Levomethorphan
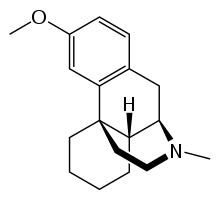
Levomethorphan (INN, BAN) is an opioid analgesic of the morphinan family that has never been marketed.[1] It is the L-stereoisomer of racemethorphan (methorphan).[1] The effects of the two isomers of the racemethorphan are quite different, with dextromethorphan (DXM) being an antitussive at low doses and a dissociative hallucinogen at much higher doses.[2] Levomethorphan is about five times stronger than morphine. [3]
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 3-6 hours |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.004.320 |
| Chemical and physical data | |
| Formula | C18H25NO |
| Molar mass | 271.404 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Levomethorphan is a prodrug to levorphanol, analogously to DXM acting as a prodrug to dextrorphan or codeine behaving as a prodrug to morphine.[4] As such, levomethorphan has similar effects to levorphanol but is less potent as it must be demethylated to the active form by liver enzymes before being able to produce its effects.[4] As a prodrug of levorphanol, levomethorphan functions as a potent agonist of all three of the opioid receptors, μ, κ (κ1 and κ3 but notably not κ2), and δ, as an NMDA receptor antagonist, and as a serotonin-norepinephrine reuptake inhibitor.[4] Via activation of the KOR, levomethorphan can produce dysphoria and psychotomimetic effects such as dissociation and hallucinations.[5]
Levomethorphan is listed under the Single Convention on Narcotic Drugs 1961 and is regulated like morphine in most countries. In the United States it is a Schedule II Narcotic controlled substance with a DEA ACSCN of 9210 and 2014 annual aggregate manufacturing quota of 195 grams, up from 6 grams the year before. The salts in use are the tartrate (free base conversion ratio 0.644) and hydrobromide (0.958).[6] At the current time, no levomethorphan pharmaceuticals are marketed in the United States.
See also
References
- Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springe. pp. 656–. ISBN 978-1-4757-2085-3.
- Hornback JM (31 January 2005). Organic Chemistry. Cengage Learning. pp. 243–. ISBN 0-534-38951-1.
- Wainer IW (1996). "Toxicology Through a Looking Glass: Stereochemical Questions and Some Answers". In Wong SH, Sunshine I (eds.). Handbook of Analytical Therapeutic Drug Monitoring and Toxicology. CRC Press.
- Gudin J, Fudin J, Nalamachu S (January 2016). "Levorphanol use: past, present and future". Postgraduate Medicine. 128 (1): 46–53. doi:10.1080/00325481.2016.1128308. PMID 26635068.
- Bruera ED, Portenoy RK (12 October 2009). Cancer Pain: Assessment and Management. Cambridge University Press. pp. 215–. ISBN 978-0-521-87927-9.
- "Conversion Factors for Controlled Substances". DEA Diversion Control Division. U.S. Department Of Justice, Drug Enforcement Administration (DEA).
| Psychedelics (5-HT2A agonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dissociatives (NMDAR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Deliriants (mAChR antagonists) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Opioids |
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Paracetamol-type |
| ||||||||||||||||
| NSAIDs |
| ||||||||||||||||
| Cannabinoids | |||||||||||||||||
| Ion channel modulators |
| ||||||||||||||||
| Myorelaxants | |||||||||||||||||
| Others | |||||||||||||||||
| |||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||