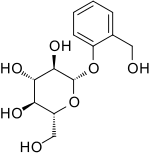
Salicin
Salicin is an alcoholic β-glucoside. Salicin is produced in (and named after) willow (Salix) bark and acts as an anti-inflammatory agent in the human body.[3] Salicin is also commonly found in the bark of Populus species, and the leaves of willows and poplars. It is also found in castoreum, which was used as an analgesic, anti-inflammatory, and antipyretic. The activity of castoreum has been credited to the accumulation of salicin from willow trees in the beaver's diet, which is transformed to salicylic acid and has an action very similar to that of aspirin.[4] Salicin was the historical origin of aspirin[5] and is chemically related to it. When consumed, the acetalic ether bridge is broken down. The two parts of the molecule, glucose and salicyl alcohol, then are metabolized separately. By oxidizing the alcohol functional group, the aromatic part finally is metabolized to salicylic acid. Salicin tastes bitter like quinine.[6]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(2R,3S,4S,5R,6S)-2-(Hydroxymethyl)-6-[2-(hydroxymethyl)phenoxy]oxane-3,
4,5-triol | |
| Other names
Salicin; D-(−)-Salicin; Salicoside; 2-(Hydroxymethyl)phenyl -β-D-glucopyranoside | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.004.847 |
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C13H18O7 | |
| Molar mass | 286.280 g·mol−1 |
| Melting point | 197 to 200 °C (387 to 392 °F; 470 to 473 K) |
| 4.3 g/100 mL | |
| Hazards | |
| Main hazards | Skin sensitizer / Contact dermatitis[2] |
| GHS pictograms |  |
| GHS Signal word | Warning |
GHS hazard statements |
H317 |
| P261, P272, P280, P302+352, P333+313, P362, P363, P501 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Salicin may cause an allergic skin reaction (skin sensitization; category 1).[2]
Mild side effects are standard, with rare occurrences of nausea, vomiting, rash, dizziness and breathing problems. Overdose from high quantities of salicin can be toxic, damaging kidneys, causing stomach ulcers, diarrhea, bleeding or digestive discomfort. Some people may be allergic or sensitive to salicylates and suffer reactions similar to those produced by aspirin. People should not take salicin if they have asthma, diabetes, gout, gastritis, hemophilia, stomach ulcers; also contraindicated are children under 16, and pregnant and breastfeeding women.[7]
References
- Merck Index, 11th Edition, 8293
- PubChem
- Uchytil, RJ (1991). "Salix drummondiana". Fire Effects Information System. Online. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory (Producer). Retrieved 2006-07-19.
- Stephen Pincock (28 March 2005). "The quest for pain relief: how much have we improved on the past?". Retrieved 2007-06-17.
- "History of Aspirin". About.com Inventors. Retrieved 2016-06-15.
- Daniells, S (2006-10-09). "Symrise explores cheaper alternatives in bitter-maskers". www.foodnavigator.com. Retrieved 2007-12-13.
- "Archived copy". Archived from the original on 2013-10-30. Retrieved 2013-10-21.CS1 maint: archived copy as title (link)
