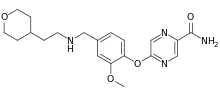
Bevenopran
Bevenopran (INN, USAN) (former developmental code names CB-5945, ADL-5945, MK-2402, OpRA III) is a peripherally acting μ-opioid receptor antagonist that also acts on δ-opioid receptors and was under development by Cubist Pharmaceuticals for the treatment of chronic opioid-induced constipation.[1][2][3] It reached phase III clinical trials for this indication before being discontinued.[4][5]
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C20H26N4O4 |
| Molar mass | 386.452 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
References
- Diego L, Atayee R, Helmons P, Hsiao G, von Gunten CF (2011). "Novel opioid antagonists for opioid-induced bowel dysfunction". Expert Opin Investig Drugs. 20 (8): 1047–56. doi:10.1517/13543784.2011.592830. PMID 21663526.
- Siemens W, Gaertner J, Becker G (2015). "Advances in pharmacotherapy for opioid-induced constipation - a systematic review". Expert Opin Pharmacother. 16 (4): 515–32. doi:10.1517/14656566.2015.995625. PMID 25539282.
- Annual Reports in Medicinal Chemistry. Elsevier Science. 13 September 2013. pp. 451–. ISBN 978-0-12-417151-0.
- Combating Opioid-Induced Constipation: New and Emerging Therapies
- Bevenopran
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.