Tribromoethanol
Tribromoethanol is an organobromine compound used as an anesthetic. It is used to anesthetize laboratory animals, particularly rodents, before surgery.[2] As a solution in tert-amyl alcohol, it has the brand name Avertin.[3] The tert-amyl alcohol acts as a weak hypnotic, in addition to improving the solubility of the tribromoethanol. Administered intravenously, tribromoethanol (Avertin) causes rapid and deep anesthesia followed by rapid and full postoperative recovery in small mammals.[4]
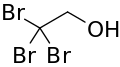 | |
| Clinical data | |
|---|---|
| Trade names | Avertin |
| Other names | Tribromoethyl alcohol |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.822 |
| Chemical and physical data | |
| Formula | C2H3Br3O |
| Molar mass | 282.757 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 73–79 °C (163–174 °F) [1] |
| Boiling point | 92–93 °C (198–199 °F) at 10 mmHg[1] |
| |
| |
In the first half of the 20th century, Avertin was also used in humans as a general anesthetic or basal narcotic to induce unconsciousness prior to the administration of other anesthetic agents. It was administered rectally as a retention enema or by intravenous injection. Its rectal use was particularly favored for pediatrics, head or neck surgery, or in mentally unstable or anxious patients.[5] Electrophysiology studies showed that tribromoethanol acts as a positive allosteric modulator of the inhibitory GABAA and glycine receptors, a mechanism similar to that seen with the related compound 2,2,2-trichloroethanol.[6] Bromal hydrate (2,2,2-tribromoethanol-1,1-diol), a compound also recognized to produce general anesthesia in animals, is metabolized to tribromoethanol.[7]
See also
References
- "2,2,2-Tribromoethanol". Sigma-Aldrich.
- "Tribromoethanol (Avertin)". Cold Spring Harbor Protocols. Cold Spring Harbor Laboratory. 2006: pdb.rec701. 2006. doi:10.1101/pdb.rec701.
- "Guidelines for the Use of Tribromoethanol/Avertin Anesthesia" (PDF). National Cancer Institute.
- Weiss J, Zimmermann F (April 1999). "Tribromoethanol (Avertin) as an anaesthetic in mice". Laboratory Animals. 33 (2): 192–3. doi:10.1258/002367799780578417. PMID 10780824.
- Edwards G (December 1945). "Tribromethyl alcohol (avertin, bromethol), 1928-1945". Proceedings of the Royal Society of Medicine. 39 (2): 71–6. doi:10.1177/003591574503900203. PMID 21010258.
- Krasowski MD, Harrison NL (February 2000). "The actions of ether, alcohol and alkane general anaesthetics on GABAA and glycine receptors and the effects of TM2 and TM3 mutations". British Journal of Pharmacology. 129 (4): 731–43. doi:10.1038/sj.bjp.0703087. PMC 1571881. PMID 10683198.
- Lehmann G, Knoefel PK (August 1938). "Trichlorethanol, tribromethanol, chloral hydrate and bromal hydrate". Journal of Pharmacology and Experimental Therapeutics. 63 (4): 453–65.