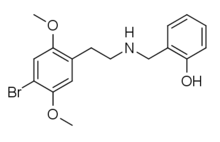
25B-NBOH
25B-NBOH (2C-B-NBOH, NBOH-2C-B) is a derivative of the phenethylamine derived hallucinogen 2C-B which has been sold as a designer drug. It acts as a potent serotonin receptor agonist with similar affinity to the better-known compound 25B-NBOMe at 5-HT2A and 5-HT2C receptors with pKis values of 8.3 and 9.4, respectively.[1][2][3][4][5][6]
 | |
| Legal status | |
|---|---|
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C17H20BrNO3 |
| Molar mass | 366.255 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Legal status
Sweden
The Riksdag added 25B-NBOH to Narcotic Drugs Punishments Act under swedish schedule I ("substances, plant materials and fungi which normally do not have medical use") as of January 26, 2016, published by Medical Products Agency (MPA) in regulation HSLF-FS 2015:35 listed as 25B-NBOH, and 2-([2-(4-bromo-2,5-dimetoxifenyl)etylamino]metyl)fenol.[7]
United Kingdom
This substance is a Class A drug in the United Kingdom as a result of the N-benzylphenethylamine catch-all clause in the Misuse of Drugs Act 1971.[8]
Analogues and derivatives
Analogues and derivatives of 2C-B:
- 2C-B-FLY
- 2CBFly-NBOMe (NBOMe-2CB-Fly)
Other:
- 2C-B-AN
- 2C-B-BUTTERFLY
- 2C-B-DragonFLY
- 2CB-5-hemifly
- 2CB-Ind
- βk-2C-B (beta-keto 2C-B)
- TCB-2 (2C-BCB)
References
- Heim R (19 March 2004). Synthese und Pharmakologie potenter 5-HT2A-Rezeptoragonisten mit N-2-Methoxybenzyl-Partialstruktur (Ph.D. thesis). Freie Universität Berlin. Archived from the original on 16 April 2004. Retrieved 27 June 2015.
- Hansen M, Phonekeo K, Paine JS, Leth-Petersen S, Begtrup M, Bräuner-Osborne H, Kristensen JL (March 2014). "Synthesis and structure-activity relationships of N-benzyl phenethylamines as 5-HT2A/2C agonists". ACS Chemical Neuroscience. 5 (3): 243–9. doi:10.1021/cn400216u. PMC 3963123. PMID 24397362.
- Hansen M (2010-12-16). Design and Synthesis of Selective Serotonin Receptor Agonists for Positron Emission Tomography Imaging of the Brain (Ph.D. thesis). University of Copenhagen. doi:10.13140/RG.2.2.33671.14245.
- Ettrup A, Hansen M, Santini MA, Paine J, Gillings N, Palner M, et al. (April 2011). "Radiosynthesis and in vivo evaluation of a series of substituted 11C-phenethylamines as 5-HT (2A) agonist PET tracers". European Journal of Nuclear Medicine and Molecular Imaging. 38 (4): 681–93. doi:10.1007/s00259-010-1686-8. PMID 21174090.
- Silva ME, Heim R, Strasser A, Elz S, Dove S (January 2011). "Theoretical studies on the interaction of partial agonists with the 5-HT2A receptor". Journal of Computer-Aided Molecular Design. 25 (1): 51–66. CiteSeerX 10.1.1.688.2670. doi:10.1007/s10822-010-9400-2. PMID 21088982.
- Braden MR, Parrish JC, Naylor JC, Nichols DE (December 2006). "Molecular interaction of serotonin 5-HT2A receptor residues Phe339(6.51) and Phe340(6.52) with superpotent N-benzyl phenethylamine agonists". Molecular Pharmacology. 70 (6): 1956–64. doi:10.1124/mol.106.028720. PMID 17000863.
- "Läkemedelsverkets föreskrifter om ändring i Läkemedelsverkets föreskrifter (LVFS 2011:10) om förteckningar över narkotik" [The Swedish Medicines Agency's regulations on amendments to the Swedish Medicines Agency's regulations (LVFS 2011: 10) on lists of drugs] (PDF). Läkemedelsverket.
- "The Misuse of Drugs Act 1971 (Ketamine etc.) (Amendment) Order 2014". www.legislation.gov.uk.
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|