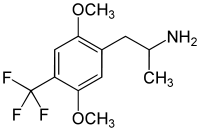
DOx
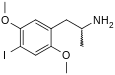
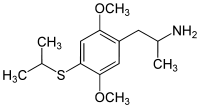
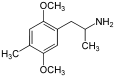
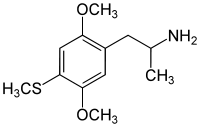
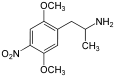
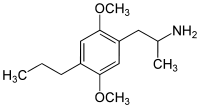
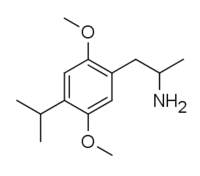
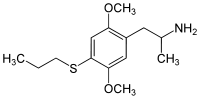
4-Substituted-2,5-dimethoxyamphetamines (DOx) is a chemical class of substituted amphetamine derivatives featuring methoxy groups at the 2- and 5- positions of the phenyl ring, and a substituent such as alkyl or halogen at the 4- position of the phenyl ring. Most compounds of this class are potent and long-lasting psychedelic drugs, and act as highly selective 5-HT2A, 5-HT2B, and 5-HT2C receptor partial agonists. A few bulkier derivatives such as DOAM have similarly high binding affinity for 5-HT2 receptors but instead act as antagonists, and so do not produce psychedelic effects.
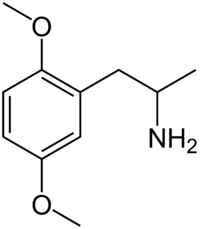
2,5-Dimethoxyamphetamine (2,5-DMA), the base structure of the DOx family
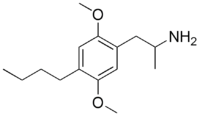
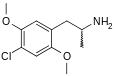
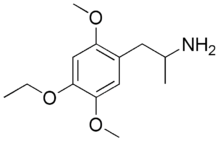
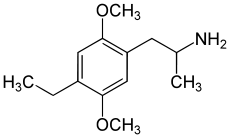
The DOx family includes the following members:
As well as the following members with additional substitutions:
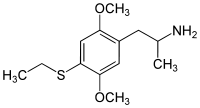
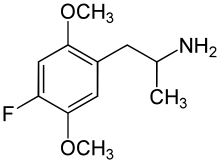
- 2,5-Dimethoxy-4-methyl-α-ethylphenethylamine (Ariadne)
- 2,5-Dimethoxy-4,N-dimethylamphetamine (Beatrice)
- 2,5-Dimethoxy-3,4-methylenedioxyamphetamine (DMMDA)
- 2,5-Dimethoxy-3,4-dimethylamphetamine (Ganesha)
- 2,5-Dimethoxy-3,4-trimethylenylamphetamine (G-3)
- 2,5-Dimethoxy-3,4-tetramethylenylamphetamine (G-4)
- 2,5-Dimethoxy-3,4-norbornylamphetamine (G-5)
- 2,5-Dimethoxy-4-iodo-N,N-dimethylamphetamine (IDNNA)
- 2,5-dimethoxy-4-bromo-N-methylamphetamine (Methyl-DOB)
- 2,3,4,5-Tetramethoxyamphetamine (TA, TeMA)
See also
References
External links
- PiHKAL ("Phenethylamines I Have Known And Loved") by Alexander "Sasha" Shulgin (1991)
- Psychotomimetic Drugs: Structure-Activity Relationships by Alexander "Sasha" Shulgin (1978)
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
Human trace amine-associated receptor ligands | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TAAR1 |
| ||||||||||
| TAAR2 |
| ||||||||||
| TAAR5 |
| ||||||||||
† References for all endogenous human TAAR1 ligands are provided at List of trace amines
‡ References for synthetic TAAR1 agonists can be found at TAAR1 or in the associated compound articles. For TAAR2 and TAAR5 agonists and inverse agonists, see TAAR for references.
| |||||||||||
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
amphetamine.svg.png)