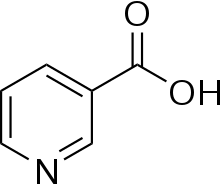
Niacin
Niacin, also known as nicotinic acid, is an organic compound and a form of vitamin B3, an essential human nutrient.[3] Niacin is obtained in the diet from a variety of whole and processed foods, with highest contents in fortified packaged foods, meat, poultry, red fish such as tuna and salmon, lesser amounts in nuts, legumes and seeds.[3][4] Niacin as a dietary supplement is used to treat pellagra, a disease caused by niacin deficiency. Signs and symptoms include skin and mouth lesions, anemia, headaches, and tiredness.[5] Many countries require its addition to wheat flour or other food grains, thereby reducing the risk of pellagra.[3][6]
| |||
| Names | |||
|---|---|---|---|
| Pronunciation | /ˈnaɪəsɪn/ | ||
| Preferred IUPAC name
Pyridine-3-carboxylic acid[1] | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol) |
|||
| 3DMet | |||
| 109591 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.401 | ||
| EC Number |
| ||
| 3340 | |||
| KEGG | |||
| MeSH | Niacin | ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C6H5NO2 | |||
| Molar mass | 123.111 g·mol−1 | ||
| Appearance | White, translucent crystals | ||
| Density | 1.473 g cm−3 | ||
| Melting point | 237 °C; 458 °F; 510 K | ||
| 18 g L−1 | |||
| log P | 0.219 | ||
| Acidity (pKa) | 2.0, 4.85 | ||
| Isoelectric point | 4.75 | ||
Refractive index (nD) |
1.4936 | ||
| 0.1271305813 D | |||
| Thermochemistry | |||
Std enthalpy of formation (ΔfH⦵298) |
−344.9 kJ mol−1 | ||
Std enthalpy of combustion (ΔcH⦵298) |
−2.73083 MJ mol−1 | ||
| Pharmacology | |||
| C04AC01 (WHO) C10BA01 (WHO) C10AD02 (WHO) C10AD52 (WHO) | |||
| License data |
| ||
| Intramuscular, by mouth | |||
| Pharmacokinetics: | |||
| 20–45 min | |||
| Hazards | |||
| GHS pictograms |  | ||
| GHS Signal word | Warning | ||
GHS hazard statements |
H319 | ||
| P264, P280, P305+351+338, P337+313, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 193 °C (379 °F; 466 K) | ||
| 365 °C (689 °F; 638 K) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682518 |
| License data |
|
| Pregnancy category | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.401 |
Niacin has the formula C
6H
5NO
2 and belongs to the group of the pyridinecarboxylic acid.[3] As the precursor for NAD and NADP, niacin is involved in DNA repair.[7]
Niacin is also a prescription medication. Amounts far in excess of the recommended dietary intake for vitamin functions will lower blood triglycerides and low density lipoprotein cholesterol (LDL-C), and raise blood high density lipoprotein cholesterol (HDL-C, often referred to as "good" cholesterol). There are two forms: immediate-release and sustained-release niacin. Initial prescription amounts are 500 mg/day, increased over time until a therapeutic effect is achieved. Immediate-release doses can be as high as 3,000 mg/day; sustained-release as high as 2,000 mg/day.[8] Despite the proven lipid changes, niacin has not been found useful for decreasing the risk of cardiovascular disease in those already on a statin.[9] A 2010 review had concluded effectiveness of niacin as a mono-therapy,[10] but a 2017 review incorporating twice as many trials concluded that prescription niacin, while affecting lipid levels, did not reduce all-cause mortality, cardiovascular mortality, myocardial infarctions, nor fatal or non-fatal strokes.[11] Prescription niacin was shown to cause hepatotoxicity[12] and increase risk of type 2 diabetes.[13][14] Niacin prescriptions in the U.S. had peaked in 2009, at 9.4 million, declining to 1.3 million by 2017.[15]
Although niacin and nicotinamide (niacinamide) are identical in their vitamin activity, nicotinamide does not have the same pharmacological, lipid-modifying effects or side effects as niacin, i.e., when niacin takes on the -amide group, it does not reduce cholesterol nor cause flushing.[16][17]
Medical uses
Treatment of deficiency
Niacin and niacinamide are used for prevention and treatment of pellagra, a disease caused by lack of the vitamin.[5] For treating deficiency, the World Health Organization (WHO) recommends administering niacinamide instead of niacin, to avoid the flushing side effect commonly caused by niacin. Guidelines suggest using 300 mg/day for three to four weeks.[18] Dementia and dermatitis show improvement within a week. Because deficiencies of other B-vitamins may be present, the WHO recommends a multi-vitamin in addition to the niacinamide.[18]
Lipids
Prescription niacin, in immediate-release and slow-release forms, is used to treat primary hyperlipidemia and mixed dyslipidemia. It is used either as a monotherapy or in combination with other lipid-modifying drugs. Dosages start at 500 mg/day and are often gradually increased to as high as 3000 mg/day for immediate release or 2000 mg/day for slow release (also referred to as sustained release) to achieve the targeted lipid changes (lower LDL-C and triglycerides, and higher HDL-C).[19][20] Prescriptions in the U.S. peaked in 2009, at 9.4 million and had declined to 1.3 million by 2017.[15] Systematic reviews found no effect of prescription niacin on all-cause mortality, cardiovascular mortality, myocardial infarctions, nor fatal or non-fatal strokes despite raising HDL cholesterol. Reported side effects include an increased risk of diabetes.[9][11][21]
Extended release niacin was combined with lovastatin as a prescription drug combination (trade name Advicor) for the treatment of dyslipidemia. It was a combination of the vitamin niacin in extended release form and the statin drug lovastatin (trade name Mevacor). The combination preparation was developed by Kos Pharmaceuticals, Inc., which was acquired by Abbott Laboratories in 2006, subsequently transferred to AbbVie Inc. when that company was spun off from Abbott in 2013. Advicor was approved by the U.S. Food and Drug Administration (FDA) on December 17, 2001.[22] Similarly, an Abbott Laboratories combination drug (trade name Simcor) consisting of an extended release form of the vitamin and the statin drug simvastatin was approved by the FDA on February 15, 2008.[23] The FDA withdrew approval of both drugs on 18 April 2016. The reason given: "Based on the collective evidence from several large cardiovascular outcome trials, the Agency has concluded that the totality of the scientific evidence no longer supports the conclusion that a drug-induced reduction in triglyceride levels and/or increase in HDL-cholesterol levels in statin-treated patients results in a reduction in the risk of cardiovascular events." AbbVie Inc. agreed to voluntarily discontinue marketing Advicor and Simcor.[24]
Contraindications
Prescription immediate release (Niacor) and extended release (Niaspan) niacin are contraindicated with active liver disease, persistent elevated serum transaminases, active peptic ulcer disease, or arterial bleeding. Also contraindicated for women who are pregnant or expecting to become pregnant, or are lactating.[25]
Adverse effects
The most common adverse effects of niacin at (50–500 mg) are flushing (e.g., warmth, redness, itching or tingling), headache, abdominal pain, diarrhea, dyspepsia, nausea, vomiting, rhinitis, pruritus and rash.[25][4] These can be minimized by initiating therapy at low dosages, increasing dosage gradually, and avoiding administration on an empty stomach.[25]
The acute adverse effects of high-dose niacin therapy (1–3 grams/day) – which is commonly used in the treatment of hyperlipidemias – further include hypotension, fatigue, glucose intolerance and insulin resistance, heartburn, blurred or impaired vision, and macular edema.[4] With long-term use, the adverse effects of high-dose niacin therapy also include hepatic dysfunction (associated with fatigue, nausea, and loss of appetite), hepatitis, and acute liver failure;[4] these hepatotoxic effects of niacin occur more often when extended-release dosage forms are used.[4] The long-term use of niacin at high doses (2 grams/day) also significantly increases the risk of cerebral hemorrhage, ischemic stroke, gastrointestinal ulceration and bleeding, diabetes, dyspepsia, and diarrhea.[4]
Facial flushing
Flushing usually lasts for about 15 to 30 minutes, though it can sometimes last up to two hours. It is sometimes accompanied by a prickly or itching sensation, in particular, in areas covered by clothing. Flushing can be blocked by taking 300 mg of aspirin half an hour before taking niacin, by taking one tablet of ibuprofen per day or by co-administering the prostaglandin receptor antagonist laropiprant. Taking niacin with meals also helps reduce this side effect. Acquired tolerance will also help reduce flushing; after several weeks of a consistent dose, most patients no longer experience flushing. Reduction of flushing focuses on altering or blocking the prostaglandin-mediated pathway.[26] Slow- or "sustained"-release forms of niacin have been developed to lessen these side effects.[27][28]
Prostaglandin (PGD2) is the primary cause of the flushing reaction, with serotonin appearing to have a secondary role in this reaction.[29] The effect is mediated by prostaglandin E2 and D2 due to GPR109A activation of epidermal Langerhans cells and keratinocytes.[30][31] Langerhans cells use cyclooxygenase type 1 (COX-1) for PGE2 production and are more responsible for acute flushing, while keratinocytes are COX-2 dependent and are in active continued vasodilation.[32][33] Flushing was often thought to involve histamine, but histamine has been shown not to be involved in the reaction.[29]
Liver damage
Niacin in medicinal doses causes elevation in serum aminotransferase in some people. The reaction is asymptomatic and usually resolves even when the drug intakes continues. The effect is dose-related. At higher doses – above three grams per day – there can also be a decrease in coagulation factors and an increase in bleeding and bruising. These changes revert to normal when therapy is stopped.[12] Niacin can also cause serious hepatotoxicity. This is uncommon, and more likely with the sustained release form of the product versus immediate release. Onset is days to weeks. Early symptoms include nausea, vomiting and abdominal pain, followed by jaundice and pruritus. Serum aminotransferase concentration is very high. The mechanism is thought to be toxicity of elevated serum niacin. Lowering dose or switching to the immediate release form can resolve symptoms. In rare instances the injury is severe, and progresses to liver failure.[12]
Diabetes
The high doses of niacin used to improve the lipid profile have been shown to elevate fasting blood glucose in people with type 2 diabetes.[13] Reviews reported that long-term niacin therapy was also associated with an increase in the risk of new-onset type 2 diabetes.[13][14]
Other
Side effects of heart arrhythmias have also been reported.[34] Increased prothrombin time and decreased platelet count have been reported; therefore, these should be monitored closely in patients who are also taking anticoagulants.[25] Extremely high doses of niacin can also cause niacin maculopathy, a thickening of the macula and retina, which leads to blurred vision and blindness. This maculopathy is reversible after niacin intake ceases.[35]
Deficiency

Plasma concentrations of other niacin metabolites and of niacin are not useful markers of niacin status. Urinary excretion of the methylated metabolite N1-methyl-nicotinamide is considered reliable and sensitive. The measurement requires a 24-hour urine collection. For adults, a value of less than 5.8 µmol/day represent deficient niacin status and 5.8 to 17.5 µmol/day represents low.[36] An alternative mean of expressing urinary N1-methyl-nicotinamide is as mg/g creatinine in a 24-hour urine collection, with deficient defined as <0.5, low 0.5-1.59, acceptable 1.6-4.29, and high >4.3[18] Niacin deficiency occurs before the signs and symptoms of pellagra appear.[36] Erythrocyte nicotinamide adenine dinucleotide (NAD) concentrations potentially provides another sensitive indicator of niacin depletion, although definitions of deficient, low, etc. have not been established. Lastly, plasma tryptophan decreases on a low niacin diet because tryptophan converts to niacin. However, low tryptophan could also be caused by a diet low in this essential amino acid.[36]
Niacin deficiency is rarely seen in developed countries, and it is more typically associated with poverty, malnutrition or chronic alcoholism.[37] It also tends to occur in less developed areas where people eat maize (corn) as a staple food, as maize is the only grain low in digestible niacin. A cooking technique called nixtamalization i.e., pretreating with alkali ingredients, increases the bioavailability of niacin during maize meal/flour production.[38] For this reason, people who consume corn as tortillas or hominy are not at risk of niacin deficiency.
Severe deficiency of niacin in the diet causes the disease pellagra, which is characterized by diarrhea, sun-sensitive dermatitis, and dementia, hyperpigmentation, thickening of the skin, inflammation of the mouth and tongue, digestive disturbances, amnesia, delirium, and eventually death, if left untreated.[5][39] Common psychiatric symptoms of niacin deficiency include irritability, poor concentration, anxiety, fatigue, restlessness, apathy, and depression.[39] The biochemical mechanism(s) for the observed neurodegeneration are not well understood, but may rest on the requirement for NAD for the tryptophan-kyneurenic acid pathway, the mitochondrial ATP generation related pathways, the poly (ADP-ibose) polymerase (PARP) pathway, the BDNF-TRKB Axis abnormalities, or changes to genome expression due to the niacin deficiency.[40] Chronic alcoholism combined with niacin deficiency may lead to psychiatric symptoms reversible with niacin supplementation.[41]
Between 1906 and 1940 more than three million Americans were affected by pellagra, with more than 100,000 deaths.[42] Joseph Goldberger was assigned to study pellagra by the Surgeon General of the United States and produced good results in diet studies conducted at orphanages.[43][44] In the late 1930s, studies by Tom Douglas Spies, Marion Blankenhorn, and Clark Cooper established that niacin cured pellagra in humans. The prevalence of the disease was greatly reduced as a result.[45]
Hartnup disease is a hereditary nutritional disorder resulting in niacin deficiency.[39] This condition was first identified in the 1950s by the Hartnup family in London. It is due to a deficit in the intestines and kidneys, making it difficult for the body to break down and absorb dietary tryptophan (an essential amino acid that is utilized to synthesize niacin). The resulting condition is similar to pellagra, including symptoms of red, scaly rash, and sensitivity to sunlight. Oral niacin is given as a treatment for this condition in doses ranging from 40–200 mg, with a good prognosis if identified and treated early.[39] Niacin synthesis is also deficient in carcinoid syndrome, because of metabolic diversion of its precursor tryptophan to form serotonin.[3]
Dietary recommendations
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The U.S. Institute of Medicine (renamed National Academy of Medicine in 2015) updated Estimated Average Requirements (EARs) and Recommended Dietary Allowances (RDAs) for niacin in 1998 (see Table).[36] The European Food Safety Authority (EFSA) refers to the collective set of information as Dietary Reference Values (DRV), with Population Reference Intake (PRI) instead of RDA, and Average Requirement instead of EAR. AI and UL defined the same as in United States. Units are milligrams per megajoule (MJ) of energy consumed. For women (including those pregnant or lactating), men and children the PRI is 1.6 mg per megajoule. As the conversion is 1 MJ = 239 kcal, an adult consuming 2390 kilocalories should be consuming 16 mg niacin. This is comparable to U.S. RDAs (14 mg/day for adult women, 16 mg/day for adult men).[49] The EFSA niacin UL is set at 10 mg/day, which is much less than the U.S. value. The UL applies to niacin as a supplement consumed as one dose, and is intended to avoid the skin flush reaction. This explains why the PRI can be higher than the UL.[50]
Both the DRI and DRV describe amounts needed as niacin equivalents (NE), calculated as 1 mg NE = 1 mg niacin or 60 mg of the essential amino acid tryptophan. This is because the amino acid is utilized to synthesize the vitamin.[36][49]
For U.S. food and dietary supplement labeling purposes the amount in a serving is expressed as a percent of Daily Value (%DV). For niacin labeling purposes 100% of the Daily Value is 16 mg. Prior to 27 May 2016 it was 20 mg, revised to bring it into agreement with the RDA.[51][52] Compliance with the updated labeling regulations was required by 1 January 2020, for manufacturers with $10 million or more in annual food sales, and by 1 January 2021 for manufacturers with less than $10 million in annual food sales.[53][54][55] During the first six months following the 1 January 2020 compliance date, the FDA plans to work cooperatively with manufacturers to meet the new Nutrition Facts label requirements and will not focus on enforcement actions regarding these requirements during that time.[53] A table of the old and new adult Daily Values is provided at Reference Daily Intake.
Sources
Niacin is found in a variety of whole and processed foods, including fortified packaged foods, meat from various animal sources, seafoods, and spices.[3][56] In general, animal-sourced foods provide about 5-10 mg niacin per serving, although dairy foods and eggs have little. Some plant-sourced foods such as nuts and grains provide about 2-5 mg niacin per serving, although this naturally present niacin is largely bound to polysaccharides and glycopeptides, making it only about 30% bioavailable. Fortified food ingredients such as wheat flour have niacin added, which is bioavailable.[4] Among whole food sources with the highest niacin content per 100 grams:
| Source[57] | Amount (mg / 100g) |
|---|---|
| Nutritional yeast[58] per 2 Tbsp (16 g) | 56 |
| Tuna, yellowfin | 22.1 |
| Peanuts | 14.3 |
| Peanut butter | 13.1 |
| Bacon | 10.4 |
| Tuna, light, canned | 10.1 |
| Salmon | 10.0 |
| Turkey depending on what part, how cooked | 7-12 |
| Chicken depending on what part, how cooked | 7-12 |
| Source[57] | Amount (mg / 100g) |
|---|---|
| Beef depending on what part, how cooked | 4-8 |
| Pork depending on what part, how cooked | 4-8 |
| Sunflower seeds | 7.0 |
| Tuna, white, canned | 5.8 |
| Almonds | 3.6 |
| Mushrooms, white | 3.6 |
| Cod fish | 2.5 |
| Rice, brown | 2.5 |
| Hot dogs | 2.0 |
| Source[57] | Amount (mg / 100g) |
|---|---|
| Avocado | 1.7 |
| Potato, baked, with skin | 1.4 |
| Corn (maize) | 1.0 |
| Rice, white | 0.5 |
| Kale | 0.4 |
| Eggs | 0.1 |
| Milk | 0.1 |
| Cheese | 0.1 |
| Tofu | 0.1 |
Vegetarian and vegan diets can provide adequate amounts if products such as nutritional yeast, peanuts, peanut butter, tahini, brown rice, mushrooms, avocado and sunflower seeds are included. Fortified foods and dietary supplements can also be consumed to insure adequate intake.[4][59]
Food preparation
Niacin naturally found in food is susceptible to destruction from high heat cooking, especially in the presence of acidic foods and sauces. It is soluble in water, and so may also be lost from foods boiled in water.[60]
Food fortification
The Food Fortification Initiative lists all countries in the world that conduct fortification programs, and within each country, what nutrients are added to which foods, and whether those programs are voluntary or mandatory.[6] As of 2019, 53 countries required food fortification of wheat flour with niacin and 14 also mandate fortification of maize flour.[61]
Pharmacology
Pharmacodynamics
Niacin and nicotinamide are both precursors of the coenzymes nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP) in vivo.[62] NAD converts to NADP by phosphorylation in the presence of the enzyme NAD+ kinase. NADP and NAD are coenzymes for many dehydrogenases, participating in many hydrogen transfer processes.[63] NAD is important in catabolism of fat, carbohydrate, protein, and alcohol, as well as cell signaling and DNA repair, and NADP mostly in anabolism reactions such as fatty acid and cholesterol synthesis.[63] High energy requirements (brain) or high turnover rate (gut, skin) organs are usually the most susceptible to their deficiency.[64]
Niacin reduces low-density lipoprotein cholesterol (LDL-C), very low-density lipoprotein cholesterol (VLDL-C), and triglycerides (TG), and increases high-density lipoprotein cholesterol (HDL-C).[65] The lipid-therapeutic effects of niacin are partly mediated through the activation of G protein-coupled receptors, including niacin receptor 1 (NIACR1) and niacin receptor 2 (NIACR2) which are highly expressed in adipose tissue, spleen, immune cells, and keratinocytes, but not in other expected organs such as liver, kidney, heart or intestine.[66][67] NIACR1 and NIACR2 inhibit cyclic adenosine monophosphate (cAMP) production and thus fat breakdown in adipose tissue and free fatty acids available for liver to produce triglycerides, VLDL-C and LDL-C.[68][69] A decrease in free fatty acids also suppresses liver expression of apolipoprotein C3 and PPARg coactivator-1b, thus increasing VLDL-C turnover and reducing its production.[70]
The mechanism behind niacin increasing HDL-C is not totally understood, but seems to occur in various ways. Niacin increases apolipoprotein A1 levels due to anticatabolic effects resulting in higher reverse cholesterol transport. It also inhibits HDL-C hepatic uptake, down-regulating production of the cholesterol ester transfer protein (CETP) gene.[65] Finally, it stimulates the ABCA1 transporter in monocytes and macrophages and upregulates peroxisome proliferator-activated receptor gamma, resulting in reverse cholesterol transport.[71]
Other effects of niacin at pharmaceutical intakes include anti-thrombosis, reduced vascular inflammation, improved endothelial function, and plaque stability.[72] As mediators produced from adipocytes, adipokines, such as tumor necrosis factor (TNF), interleukins and chemokines, have pro-inflammatory effects, while others, such as adiponectin, have anti-inflammatory effects that influence the onset of atherosclerosis.[73]
Research has been able to show the function of niacin in the lipid metabolism pathway. It is seen that this vitamin can decrease the synthesis of apoB-containing lipoproteins such as VLDL-C, LDL-C, IDL and lipoprotein (a) via several mechanisms: (1) directly inhibiting the action of DGAT2, a key enzyme for triglyceride synthesis; (2) influencing binding to the receptor HCAR2 thereby decreasing lipolysis and FFA flux to the liver for triglyceride synthesis; and (3) increasing apoB catabolism. HDL levels are increased by niacin through direct and indirect pathways, such as by decreasing cholesterylester transfer protein activity and triglyceride levels.[74]
Pharmacokinetics
Both niacin and niacinamide are rapidly absorbed from the stomach and small intestine. Absorption is facilitated by sodium-dependent diffusion, and at higher intakes, via passive diffusion. Unlike some other vitamins, the percent absorbed does not decrease with increasing dose, so that even at amounts of 3-4 grams, absorption is nearly complete.[75] Niacinamide is the major form in the bloodstream. In the liver, niacinamide is converted to storage nicotinamide adenine dinucleotide (NAD). As needed, liver NAD is hydrolyzed to niacinamide and niacin for transport to tissues, there reconverted to NAD to serve as an enzyme cofactor.[75] Excess niacin is methylated in the liver to N1-methylnicotinamide (NMN) and excreted in urine as such or as the oxidized metabolite N1-methyl-2-pyridone-5-carboxamide (2-pyridone). Decreased urinary content of these metabolites is a measure of niacin deficiency.[75]

Production
Biosynthesis
In all animals the liver can synthesize niacin from the essential amino acid tryptophan, a five-step process with the penultimate compound being quinolinic acid (see figure). Some bacteria and plants utilize aspartic acid in a pathway that also goes to quinolinic acid.[76] For humans, the efficiency of conversion is estimated as requiring 60 mg of tryptophan to make 1 mg of niacin. Riboflavin, vitamin B6 and iron are required for the process. The US Institute of Medicine set a Recommended Dietary Allowance (RDA) for adults of 5 mg/kg body weight/day for tryptophan,[77] equivalent to 350 mg/day for a 70 kg (154 lb) adult. The RDA for protein is 0.8 g/kg, equivalent to 56 g/day for a 70 kg adult. Dietary protein is approximately 1% tryptophan,[75] so achieving the RDA for protein contributes about 560 mg tryptophan per day.[77] Pellagra, a disease due to niacin deficiency, was observed in Europe and elsewhere when poor people consumed large amounts of corn as part of their protein-poor diet, but not so for those in areas where wheat or rice were the low-cost grain, the reason being that corn protein has a lower tryptophan content.[78]
Industrial synthesis
Commercial production for animal feed and other purposes is of nicotinamide (niacinamide), which can be converted to niacin. Nicotinonitrile is produced by ammoxidation of 3-methylpyridine. Nitrile hydratase is then used to catalyze nicotinonitrile to nicotinamide.[79] According to Ullmann's Encyclopedia of Industrial Chemistry, worldwide 31,000 tons of nicotinamide were sold in 2014.[80]
Chemistry
This colorless, water-soluble solid is a derivative of pyridine, with a carboxyl group (COOH) at the 3-position. Other forms of vitamin B3 include the corresponding amide nicotinamide (niacinamide), where the carboxyl group has been replaced by a carboxamide group (CONH
2), as well as more complex amides and a variety of esters.
Preparations
Niacin is available as a prescription product, and in the United States as a dietary supplement. Prescription products can be immediate release (Niacor, 500 mg tablets) or extended release (Niaspan, 500 and 1000 mg tablets). Dietary supplement products can be immediate or slow release, the latter including inositol hexanicotinate.[81][82] The last has questionable clinical efficacy in reducing cholesterol levels.[83]
Nicotinamide
Nicotinamide may be obtained from the diet where it is present primarily as NAD+ and NADP+. These are hydrolysed in the intestine and the resulting nicotinamide is absorbed either as such, or following its hydrolysis, to niacin. Nicotinamide may be toxic to the liver at doses exceeding 3 g/day for adults.[84]
Extended release
A prescription extended release niacin, Niaspan, has a film coating that delays release of the niacin, resulting in an absorption over a period of 8–12 hours. The extended release formulations generally reduce vasodilation and flushing side effects, but increase the risk of hepatotoxicity compared to the immediate release forms.[85][86]
A combination of niacin and laropiprant had been approved for use in Europe and marketed as Tredaptive. Laropiprant is a prostaglandin D2 binding drug shown to reduce vasodilation and flushing up to 73%.[65][87][88] A clinical trial showed no additional efficacy of Tredaptive in lowering cholesterol when used together with other statin drugs, but did show an increase in other side effects.[89] The study resulted in the withdrawal of Tredaptive from the international market.[90][91]
Inositol hexanicotinate
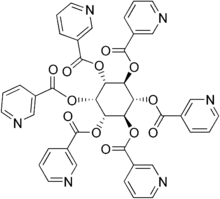
One form of dietary supplement is inositol hexanicotinate (IHN), also called inositol nicotinate, which is inositol that has been esterified with niacin on all six of inositol's alcohol groups.[92] IHN is usually sold as "flush-free" or "no-flush" niacin in units of 250, 500, or 1000 mg/tablets or capsules. In the US, it is sold as an over-the-counter formulation, and often is marketed and labeled as niacin, thus misleading consumers into thinking they are getting an active form of the medication. While this form of niacin does not cause the flushing associated with the immediate-release products, the evidence that it has lipid-modifying functions is disputed. As most of the clinical trials date from the early 1960s or the late 1970s, it is difficult to assess them by today's standards.[83][93][94] Thus, so far there is not enough evidence to recommend IHN to treat dyslipidemia.[93][94]
Renaming
In 1942, when flour enrichment with nicotinic acid began, a headline in the popular press said "Tobacco in Your Bread." In response, the Council on Foods and Nutrition of the American Medical Association approved of the Food and Nutrition Board's new names niacin and niacin amide for use primarily by non-scientists. It was thought appropriate to choose a name to dissociate nicotinic acid from nicotine, to avoid the perception that vitamins or niacin-rich food contains nicotine, or that cigarettes contain vitamins.[95] The resulting name niacin was derived from nicotinic acid + vitamin.[18][96]
History
Niacin was first described by chemist Hugo Weidel in 1873 in his studies of nicotine.[97] The original preparation remains useful: the oxidation of nicotine using nitric acid.[98] For the first time, niacin was extracted by Casimir Funk, but he thought that it was thiamine and due to the discovered amine group he coined the term "vitamine". Niacin was extracted from livers by biochemist Conrad Elvehjem in 1937, who later identified the active ingredient, then referred to as the "pellagra-preventing factor" and the "anti-blacktongue factor."[99] Soon after, in studies conducted in Alabama and Cincinnati, Dr. Tom Spies found that nicotinic acid cured the sufferers of pellagra.[44]
Niacin is referred to as vitamin B3 because it was the third of the B vitamins to be discovered. It has historically been referred to as "vitamin PP", "vitamin P-P" and "PP-factor", that are derived from the term "pellagra-preventive factor".[18] Carpenter found in 1951, that niacin in corn is biologically unavailable, and can be released only in very alkaline lime water of pH 11.[100] In 1955, Altschul and colleagues described niacin as having a lipid-lowering property.[101] As such, niacin is the oldest known lipid-lowering drug.[102]
Research
In animal models and in vitro, niacin produces marked anti-inflammatory effects in a variety of tissues – including the brain, gastrointestinal tract, skin, and vascular tissue – through the activation of hydroxycarboxylic acid receptor 2 (HCA2), also known as niacin receptor 1 (NIACR1).[103][104][105][106] Niacin has been shown to attenuate neuroinflammation and may have efficacy in treating neuroimmune disorders.[103][106] Unlike niacin, nicotinamide does not activate NIACR1; however, both niacin and nicotinamide activate the G protein-coupled estrogen receptor (GPER) in vitro.[107]
In 2014, concurring with earlier work in 2001, by Arizona State University, researchers from Pennsylvania State University working with NASA found niacin, pyridine carboxylic acids and pyridine dicarboxylic acids inside meteorites.[108]
References
- "Chapter P-6. Applications to Specific Classes of Compounds". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: Royal Society of Chemistry. 2014. pp. 648–1047. doi:10.1039/9781849733069-00648. ISBN 978-0-85404-182-4.
- "Niacin Use During Pregnancy". Drugs.com. 29 July 2019. Retrieved 4 May 2020.
- "Niacin". Micronutrient Information Center, Linus Pauling Institute, Oregon State University, Corvallis, OR. 8 October 2018. Retrieved 16 September 2019.
- "Niacin Fact Sheet for Health Professionals". 15 January 2019. Retrieved 20 January 2019. Cite journal requires
|journal=(help) - Hegyi J, Schwartz RA, Hegyi V (January 2004). "Pellagra: dermatitis, dementia, and diarrhea". International Journal of Dermatology. 43 (1): 1–5. doi:10.1111/j.1365-4632.2004.01959.x. PMID 14693013.
- "Why fortify?". Food Fortification Initiative. 2017. Retrieved 4 April 2017.
- Kennedy DO (January 2016). "B Vitamins and the Brain: Mechanisms, Dose and Efficacy—A Review". Nutrients. 8 (2): 68. doi:10.3390/nu8020068. PMC 4772032. PMID 26828517.
- "Niacin". Drugs.com. 16 March 2019. Retrieved 27 April 2020.
- Keene D, Price C, Shun-Shin MJ, Francis DP (July 2014). "Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: meta-analysis of randomised controlled trials including 117,411 patients". BMJ. 349: g4379. doi:10.1136/bmj.g4379. PMC 4103514. PMID 25038074.
- Bruckert E, Labreuche J, Amarenco P (June 2010). "Meta-analysis of the effect of nicotinic acid alone or in combination on cardiovascular events and atherosclerosis". Atherosclerosis. 210 (2): 353–61. doi:10.1016/j.atherosclerosis.2009.12.023. PMID 20079494.
- Schandelmaier S, Briel M, Saccilotto R, Olu KK, Arpagaus A, Hemkens LG, Nordmann AJ (June 2017). "Niacin for primary and secondary prevention of cardiovascular events". The Cochrane Database of Systematic Reviews. 6: CD009744. doi:10.1002/14651858.CD009744.pub2. PMC 6481694. PMID 28616955.
- "Niacin". IN: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury (Internet). Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases. February 2014. PMID 31643504.
- Ong KL, Barter PJ, Waters DD (April 2014). "Cardiovascular drugs that increase the risk of new-onset diabetes". Am. Heart J. 167 (4): 421–8. doi:10.1016/j.ahj.2013.12.025. PMID 24655688.
- Goldie C, Taylor AJ, Nguyen P, McCoy C, Zhao XQ, Preiss D (February 2016). "Niacin therapy and the risk of new-onset diabetes: a meta-analysis of randomised controlled trials". Heart. 102 (3): 198–203. doi:10.1136/heartjnl-2015-308055. PMC 4752613. PMID 26370223.
- "Niacin - Drug Usage Statistics". ClinCalc. Retrieved 11 April 2020.
- Jaconello P (October 1992). "Niacin versus niacinamide". CMAJ. 147 (7): 990. PMC 1336277. PMID 1393911.
- Kirkland JB (May 2012). "Niacin requirements for genomic stability". Mutation Research. 733 (1–2): 14–20. doi:10.1016/j.mrfmmm.2011.11.008. PMID 22138132.
- World Health Organization (2000). "Pellagra And Its Prevention And Control In Major Emergencies". World Health Organization (WHO). hdl:10665/66704. WHO/NHD/00.10. Cite journal requires
|journal=(help) - "NIACOR-niacin tablet". DAILYMED, US National Library of Medicine. March 2020. Retrieved 9 May 2020.
- "NIASPAN Patient Package and Product Information (PPPI)" (PDF). December 2018. Retrieved 9 May 2020.
- Garg A, Sharma A, Krishnamoorthy P, Garg J, Virmani D, Sharma T, Stefanini G, Kostis JB, Mukherjee D, Sikorskaya E (February 2017). "Role of Niacin in Current Clinical Practice: A Systematic Review". The American Journal of Medicine. 130 (2): 173–187. doi:10.1016/j.amjmed.2016.07.038. PMID 27793642.
- "Advicor (Niacin Extended-Release & Lovastatin) Tablets". U.S. Food and Drug Administration; Drug Approval Package. 13 September 2002. Retrieved 17 May 2020.
- "Drugs.com, Abbott Receives FDA Approval for Simcor (Niaspan / simvastatin), a Novel Combination Medicine for Comprehensive Cholesterol Management". Retrieved 15 March 2008.
- "AbbVie Inc.; Withdrawal of Approval of New Drug Applications for ADVICOR and SIMCOR". U.S. Federal Register. 18 April 2016. Retrieved 17 May 2020.
- Niaspan® (niacin extended-release) tablets prescribing information. AbbVie Inc., US-NIAS-180036, North Chicago, IL 60064 December 2018
- Kamanna VS, Kashyap ML (April 2008). "Mechanism of action of niacin". The American Journal of Cardiology. 101 (8A): 20B–26B. doi:10.1016/j.amjcard.2008.02.029. PMID 18375237.
- Katzung, Bertram G. (2006). Basic and clinical pharmacology. New York: McGraw-Hill Medical Publishing Division. ISBN 978-0-07-145153-6.
- Barter, P (October 2006). "Options for therapeutic intervention: How effective are the different agents?". European Heart Journal Supplements. 8 (F): F47–F53. doi:10.1093/eurheartj/sul041.
- Papaliodis D, Boucher W, Kempuraj D, Michaelian M, Wolfberg A, House M, Theoharides TC (December 2008). "Niacin-induced "flush" involves release of prostaglandin D2 from mast cells and serotonin from platelets: evidence from human cells in vitro and an animal model". The Journal of Pharmacology and Experimental Therapeutics. 327 (3): 665–72. doi:10.1124/jpet.108.141333. PMID 18784348. S2CID 5609632.
- Benyó Z, Gille A, Kero J, Csiky M, Suchánková MC, Nüsing RM, Moers A, Pfeffer K, Offermanns S (December 2005). "GPR109A (PUMA-G/HM74A) mediates nicotinic acid-induced flushing". The Journal of Clinical Investigation. 115 (12): 3634–40. doi:10.1172/JCI23626. PMC 1297235. PMID 16322797.
- Benyó Z, Gille A, Bennett CL, Clausen BE, Offermanns S (December 2006). "Nicotinic acid-induced flushing is mediated by activation of epidermal langerhans cells". Molecular Pharmacology. 70 (6): 1844–9. doi:10.1124/mol.106.030833. PMID 17008386. S2CID 30199951.
- Hanson J, Gille A, Zwykiel S, Lukasova M, Clausen BE, Ahmed K, Tunaru S, Wirth A, Offermanns S (August 2010). "Nicotinic acid- and monomethyl fumarate-induced flushing involves GPR109A expressed by keratinocytes and COX-2-dependent prostanoid formation in mice". The Journal of Clinical Investigation. 120 (8): 2910–9. doi:10.1172/JCI42273. PMC 2912194. PMID 20664170.
- Maciejewski-Lenoir D, Richman JG, Hakak Y, Gaidarov I, Behan DP, Connolly DT (December 2006). "Langerhans cells release prostaglandin D2 in response to nicotinic acid". The Journal of Investigative Dermatology. 126 (12): 2637–46. doi:10.1038/sj.jid.5700586. PMID 17008871.
- Brunton LL, Lazo JS, Parker K, eds. (2005). Goodman & Gilman's The Pharmacological Basis of Therapeutics (11th ed.). New York: McGraw-Hill. ISBN 978-0-07-142280-2.
- Domanico D, Verboschi F, Altimari S, Zompatori L, Vingolo EM (2015). "Ocular Effects of Niacin: A Review of the Literature". Med Hypothesis Discov Innov Ophthalmol. 4 (2): 64–71. PMC 4458328. PMID 26060832.
- Institute of Medicine (1998). "Niacin". Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: The National Academies Press. pp. 123–149. ISBN 978-0-309-06554-2. Retrieved 29 August 2018.
- Pitsavas S, Andreou C, Bascialla F, Bozikas VP, Karavatos A (March 2004). "Pellagra encephalopathy following B-complex vitamin treatment without niacin". International Journal of Psychiatry in Medicine. 34 (1): 91–5. doi:10.2190/29XV-1GG1-U17K-RGJH. PMID 15242145. S2CID 29070525.
- Bressani R, Gomez-Brenes R, Scrimshaw NS (1961). "Effect of processing on distribution and in vitro availability of niacin of corn (Zea mays)". Food Technol. 15: 450–4.
- Prakash R, Gandotra S, Singh LK, Das B, Lakra A (2008). "Rapid resolution of delusional parasitosis in pellagra with niacin augmentation therapy". General Hospital Psychiatry. 30 (6): 581–4. doi:10.1016/j.genhosppsych.2008.04.011. PMID 19061687.
- Fu L, Doreswamy V, Prakash R (August 2014). "The biochemical pathways of central nervous system neural degeneration in niacin deficiency". Neural Regeneration Research. 9 (16): 1509–13. doi:10.4103/1673-5374.139475. PMC 4192966. PMID 25317166.
- Badawy AA (May 2014). "Pellagra and alcoholism: a biochemical perspective". Alcohol Alcohol. 49 (3): 238–50. doi:10.1093/alcalc/agu010. PMID 24627570.
- Rajakumar, Kumaravel (2000). "Pellagra in the United States: A Historical Perspective" (PDF). Southern Medical Journal. 93 (3): 272–7. doi:10.1097/00007611-200093030-00005. PMID 10728513. Archived (PDF) from the original on 24 February 2015.
- Evans BK; Feinstein AR. (September 1994). "Joseph Goldberger: an unsung hero of American clinical epidemiology". Ann Intern Med. 121 (5): 372–75. doi:10.7326/0003-4819-121-5-199409010-00010. PMID 8042827. S2CID 13226008.
- Kraut A. "Dr. Joseph Goldberger and the War on Pellagra | Ashes on the Potomac". history.nih.gov. Retrieved 20 February 2017.
- Ruth Hanna Sachs, White Rose History. Volume I. 2003. Appendix D, p. 2 ISBN 978-0-9710541-9-6 "Men of the Year, outstanding in comprehensive science were three medical researchers who discovered that nicotinic acid was a cure for human pellagra: Drs. Tom Douglas Spies of Cincinnati General Hospital, Marion Arthur Blankenhorn of the University of Cincinnati, Clark Niel Cooper of Waterloo, Iowa."
- "Nutrient reference values for Australia and New Zealand" (PDF). National Health and Medical Research Council. 9 September 2005. Archived from the original (PDF) on 21 January 2017. Retrieved 19 June 2018.
- Health Canada (20 July 2005). "Dietary Reference Intakes". Government of Canada. Retrieved 20 June 2018.
- "Tolerable Upper Intake Levels for Vitamins and Minerals" (PDF). European Food Safety Authority. February 2006. Retrieved 18 June 2018.
- "Overview on Dietary Reference Values for the EU population as derived by the EFSA Panel on Dietetic Products, Nutrition and Allergies" (PDF). 2017.
- "Tolerable Upper Intake Levels For Vitamins And Minerals" (PDF). European Food Safety Authority. 2006.
- "Federal Register May 27, 2016 Food Labeling: Revision of the Nutrition and Supplement Facts Labels" (PDF).
- "Daily Value Reference of the Dietary Supplement Label Database (DSLD)". Dietary Supplement Label Database (DSLD). Retrieved 16 May 2020.
- "FDA provides information about dual columns on Nutrition Facts label". U.S. Food and Drug Administration (FDA). 30 December 2019. Retrieved 16 May 2020.

- "Changes to the Nutrition Facts Label". U.S. Food and Drug Administration (FDA). 27 May 2016. Retrieved 16 May 2020.

- "Industry Resources on the Changes to the Nutrition Facts Label". U.S. Food and Drug Administration (FDA). 21 December 2018. Retrieved 16 May 2020.

- "Niacin content per 100 grams; select food subset, abridged list by food groups". United States Department of Agriculture, Agricultural Research Service, USDA Branded Food Products Database v.3.6.4.1. 17 January 2017. Retrieved 23 January 2017.
- "USDA National Nutrient Database for Standard Reference Legacy: Niacin" (PDF). U.S. Department of Agriculture, Agricultural Research Service. 2018. Retrieved 12 May 2020.
- "Nutritional Yeast Flakes (two tablespoons = 16 grams". NutritionData.Self.com. Retrieved 13 May 2020.
- "Vitamin B3 (Niacin)". VivaHealth.org. 2000. Retrieved 12 May 2020.
- "Effects of Cooking on Vitamins (Table)". Beyondveg. Archived from the original on 16 October 2012. Retrieved 30 April 2019.
- "Map: Count of Nutrients In Fortification Standards". Global Fortification Data Exchange. Retrieved 27 April 2020.
- Cox M, Lehninger AL, Nelson DR (2000). Lehninger principles of biochemistry. New York: Worth Publishers. ISBN 978-1-57259-153-0.
- Wan P, Moat S, Anstey A (June 2011). "Pellagra: a review with emphasis on photosensitivity". The British Journal of Dermatology. 164 (6): 1188–200. doi:10.1111/j.1365-2133.2010.10163.x. PMID 21128910.
- Ishii N, Nishihara Y (March 1981). "Pellagra among chronic alcoholics: clinical and pathological study of 20 necropsy cases". Journal of Neurology, Neurosurgery, and Psychiatry. 44 (3): 209–15. doi:10.1136/jnnp.44.3.209. PMC 490893. PMID 7229643.
- Villines TC, Kim AS, Gore RS, Taylor AJ (February 2012). "Niacin: the evidence, clinical use, and future directions". Current Atherosclerosis Reports. 14 (1): 49–59. doi:10.1007/s11883-011-0212-1. PMID 22037771. S2CID 27925461.
- Soga T, Kamohara M, Takasaki J, Matsumoto S, et al. (March 2003). "Molecular identification of nicotinic acid receptor". Biochemical and Biophysical Research Communications. 303 (1): 364–9. doi:10.1016/S0006-291X(03)00342-5. PMID 12646212.
- Wise A, Foord SM, Fraser NJ, Barnes AA, et al. (March 2003). "Molecular identification of high and low affinity receptors for nicotinic acid". The Journal of Biological Chemistry. 278 (11): 9869–74. doi:10.1074/jbc.M210695200. PMID 12522134.
- Gille A, Bodor ET, Ahmed K, Offermanns S (2008). "Nicotinic acid: pharmacological effects and mechanisms of action". Annual Review of Pharmacology and Toxicology. 48 (1): 79–106. doi:10.1146/annurev.pharmtox.48.113006.094746. PMID 17705685.
- Wanders D, Judd RL (August 2011). "Future of GPR109A agonists in the treatment of dyslipidaemia". Diabetes, Obesity & Metabolism. 13 (8): 685–91. doi:10.1111/j.1463-1326.2011.01400.x. PMID 21418500.
- Hernandez C, Molusky M, Li Y, Li S, Lin JD (October 2010). "Regulation of hepatic ApoC3 expression by PGC-1β mediates hypolipidemic effect of nicotinic acid". Cell Metabolism. 12 (4): 411–9. doi:10.1016/j.cmet.2010.09.001. PMC 2950832. PMID 20889132.
- Rubic T, Trottmann M, Lorenz RL (February 2004). "Stimulation of CD36 and the key effector of reverse cholesterol transport ATP-binding cassette A1 in monocytoid cells by niacin". Biochemical Pharmacology. 67 (3): 411–9. doi:10.1016/j.bcp.2003.09.014. PMID 15037193.
- Wu BJ, Yan L, Charlton F, Witting P, Barter PJ, Rye KA (May 2010). "Evidence that niacin inhibits acute vascular inflammation and improves endothelial dysfunction independent of changes in plasma lipids". Arteriosclerosis, Thrombosis, and Vascular Biology. 30 (5): 968–75. doi:10.1161/ATVBAHA.109.201129. PMID 20167660.
- Gustafson B (April 2010). "Adipose tissue, inflammation and atherosclerosis". Journal of Atherosclerosis and Thrombosis. 17 (4): 332–41. doi:10.5551/jat.3939. PMID 20124732.
- Creider JC, Hegele RA, Joy TR (September 2012). "Niacin: another look at an underutilized lipid-lowering medication". Nature Reviews. Endocrinology. 8 (9): 517–28. doi:10.1038/nrendo.2012.22. PMID 22349076. S2CID 22526314.
- Pemberthy, WT; Kirkland, JB (2012). "Niacin". In JW Erdman Jr, IA MacDonald, SH Zeisel (eds.). Present Knowledge in Nutrition, Tenth Edition. Hoboken, NJ: Wiley-Blackwell. pp. 293–306. ISBN 978-0-470-95917-6.CS1 maint: uses editors parameter (link)
- Foster JW, Moat AG (1 March 1980). "Nicotinamide adenine dinucleotide biosynthesis and pyridine nucleotide cycle metabolism in microbial systems". Microbiol. Rev. 44 (1): 83–105. doi:10.1128/MMBR.44.1.83-105.1980. PMC 373235. PMID 6997723.
- Institute of Medicine (2002). "Protein and Amino Acids". Dietary Reference Intakes for Energy, Carbohydrates, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC: The National Academies Press. pp. 589–768.
- Carpenter KJ (1983). "The relationship of pellagra to corn and the low availability of niacin in cereals". Experientia Suppl. Experientia Supplementum. 44: 197–222. doi:10.1007/978-3-0348-6540-1_12. ISBN 978-3-0348-6542-5. PMID 6357846.
- Abe N, Ichimura H, Kataoka T, Morishita S, Shimizu S, Shoji T, Watanabe N (2007). "Pyridine and Pyridine Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a22_399.
- Blum, René (2015). "Vitamins, 11. Niacin (Nicotinic Acid, Nicotinamide)". Vitamins, 11. Niacin (Nicotinic Acid, Nicotinamide. Ullmann's Encyclopedia of Industrial Chemistry (6th ed.). Weinheim: Wiley-VCH. pp. 1–9. doi:10.1002/14356007.o27_o14.pub2. ISBN 978-3-527-30385-4.
- Dunatchik AP, Ito MK, Dujovne CA (1 March 2012). "A systematic review on evidence of the effectiveness and safety of a wax-matrix niacin formulation". Journal of Clinical Lipidology. 6 (2): 121–31. doi:10.1016/j.jacl.2011.07.003. PMID 22385545.
- Meyers CD, Carr MC, Park S, Brunzell JD (December 2003). "Varying cost and free nicotinic acid content in over-the-counter niacin preparations for dyslipidemia". Annals of Internal Medicine. 139 (12): 996–1002. CiteSeerX 10.1.1.694.2773. doi:10.7326/0003-4819-139-12-200312160-00009. PMID 14678919. S2CID 23980567.
- Keenan JM (1 January 2013). "Wax-matrix extended-release niacin vs inositol hexanicotinate: a comparison of wax-matrix, extended-release niacin to inositol hexanicotinate "no-flush" niacin in persons with mild to moderate dyslipidemia". Journal of Clinical Lipidology. 7 (1): 14–23. doi:10.1016/j.jacl.2012.10.004. PMID 23351578.
- Knip M, Douek IF, Moore WP, Gillmor HA, McLean AE, Bingley PJ, Gale EA (November 2000). "Safety of high-dose nicotinamide: a review". Diabetologia. 43 (11): 1337–45. doi:10.1007/s001250051536. PMID 11126400.
- Bassan M (2012). "A case for immediate-release niacin". Heart & Lung. 41 (1): 95–8. doi:10.1016/j.hrtlng.2010.07.019. PMID 21414665.
- Reiche I, Westphal S, Martens-Lobenhoffer J, Tröger U, Luley C, Bode-Böger SM (January 2011). "Pharmacokinetics and dose recommendations of Niaspan® in chronic kidney disease and dialysis patients". Nephrology, Dialysis, Transplantation. 26 (1): 276–82. doi:10.1093/ndt/gfq344. PMID 20562093.
- Lai E, De Lepeleire I, Crumley TM, Liu F, Wenning LA, Michiels N, Vets E, O'Neill G, Wagner JA, Gottesdiener K (June 2007). "Suppression of niacin-induced vasodilation with an antagonist to prostaglandin D2 receptor subtype 1". Clinical Pharmacology and Therapeutics. 81 (6): 849–57. doi:10.1038/sj.clpt.6100180. PMID 17392721.
- Paolini JF, Bays HE, Ballantyne CM, Davidson M, Pasternak R, Maccubbin D, Norquist JM, Lai E, Waters MG, Kuznetsova O, Sisk CM, Mitchel YB (November 2008). "Extended-release niacin/laropiprant: reducing niacin-induced flushing to better realize the benefit of niacin in improving cardiovascular risk factors". Cardiology Clinics. 26 (4): 547–60. doi:10.1016/j.ccl.2008.06.007. PMID 19031552.
- Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, Wallendszus K, Craig M, Jiang L, Collins R, Armitage J (July 2014). "Effects of extended-release niacin with laropiprant in high-risk patients". N. Engl. J. Med. 371 (3): 203–12. doi:10.1056/NEJMoa1300955. PMID 25014686.
- Nainggolan L (11 January 2013). "Niacin/Laropiprant Products to Be Suspended Worldwide". Medscape. Retrieved 20 February 2017.
- "Merck begins overseas recall of HDL cholesterol drug". Reuters. 11 January 2013.
- Aguilar F, Charrondiere UR, Dusemund B, Galtier PM, Gilbert J, Gott DM, et al. (January 2009). "Inositol hexanicotinate (inositol hexaniacinate) as a source of niacin (vitamin B3) added for nutritional purposes in food supplements". The EFSA Journal. 949: 1–20.
- Taheri, R (15 January 2003). "No-Flush Niacin for the Treatment of Hyperlipidemia". Medscape. Retrieved 31 March 2008.
- Meyers CD, Carr MC, Park S, Brunzell JD (December 2003). "Varying cost and free nicotinic acid content in over-the-counter niacin preparations for dyslipidemia". Annals of Internal Medicine. 139 (12): 996–1002. CiteSeerX 10.1.1.694.2773. doi:10.7326/0003-4819-139-12-200312160-00009. PMID 14678919. S2CID 23980567.
- "Niacin and Nicotinic Acid". Journal of the American Medical Association. 118 (10): 823. 7 March 1942. doi:10.1001/jama.1942.02830100053014.
- "Niacin and Niacin Amide". Journal of the American Medical Association. 118 (10): 819. 7 March 1942. doi:10.1001/jama.1942.02830100049011.
- Weidel, H (1873). "Zur Kenntniss des Nicotins". Justus Liebigs Annalen der Chemie und Pharmacie. 165 (2): 330–49. doi:10.1002/jlac.18731650212.
- Samuel M. McElvain (1941). "Nicotinic Acid". Organic Syntheses.; Collective Volume, 1, p. 385
- Elvehjem CA, Madden RJ, Strongandd FM, Woolley DW (1938). "The isolation and identification of the anti-blacktongue factor J" (PDF). J. Biol. Chem. 123 (1): 137–49.
- Laguna J, Carpenter KJ (September 1951). "Raw versus processed corn in niacin-deficient diets". The Journal of Nutrition. 45 (1): 21–8. doi:10.1093/jn/45.1.21. PMID 14880960.
- Altschul R, Hoffer A, Stephen JD (February 1955). "Influence of nicotinic acid on serum cholesterol in man". Archives of Biochemistry and Biophysics. 54 (2): 558–9. doi:10.1016/0003-9861(55)90070-9. PMID 14350806.
- Romani M, Hofer DC, Katsyuba E, Auwerx J (April 2019). "Niacin: an old lipid drug in a new NAD+ dress". J. Lipid Res. 60 (4): 741–6. doi:10.1194/jlr.S092007. PMC 6446705. PMID 30782960.
- Offermanns S, Schwaninger M (April 2015). "Nutritional or pharmacological activation of HCA(2) ameliorates neuroinflammation". Trends in Molecular Medicine. 21 (4): 245–55. doi:10.1016/j.molmed.2015.02.002. PMID 25766751.
- Chai JT, Digby JE, Choudhury RP (May 2013). "GPR109A and vascular inflammation". Current Atherosclerosis Reports. 15 (5): 325. doi:10.1007/s11883-013-0325-9. PMC 3631117. PMID 23526298.
- Graff EC, Fang H, Wanders D, Judd RL (February 2016). "Anti-inflammatory effects of the hydroxycarboxylic acid receptor 2". Metabolism. 65 (2): 102–13. doi:10.1016/j.metabol.2015.10.001. PMID 26773933.
- Wakade C, Chong R (December 2014). "A novel treatment target for Parkinson's disease". Journal of the Neurological Sciences. 347 (1–2): 34–8. doi:10.1016/j.jns.2014.10.024. PMID 25455298. S2CID 29760853.
- Santolla MF, De Francesco EM, Lappano R, Rosano C, Abonante S, Maggiolini M (July 2014). "Niacin activates the G protein estrogen receptor (GPER)-mediated signalling". Cellular Signalling. 26 (7): 1466–75. doi:10.1016/j.cellsig.2014.03.011. PMID 24662263.
- "Vitamin B3 Might Have Been Made in Space, Delivered to Earth by Meteorites". NASA. 17 April 2014. Retrieved 27 April 2018.