Neramexane
Neramexane is a drug related to memantine,[1] which acts as an NMDA antagonist[2] and has neuroprotective effects.[3] It is being developed for various possible applications, including treatment of tinnitus,[4][5] Alzheimer's disease,[6] drug addiction[7] and as an analgesic.[8] Animal studies have also suggested antidepressant[9] and nootropic[10] actions, so there are a wide range of potential applications this drug may be used for. It also acts as a nicotinic acetylcholine receptor antagonist.[11]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.107.752 |
| Chemical and physical data | |
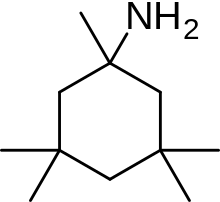
| Formula | C11H23N |
| Molar mass | 169.312 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
A clinical trial found that doses of 50 mg and above safely improved tinnitus scores over 16 weeks.[12]
See also
References
- Gilling, K; Jatzke, C; Wollenburg, C; Vanejevs, M; Kauss, V; Jirgensons, A; Parsons, CG (2007). "A novel class of amino-alkylcyclohexanes as uncompetitive, fast, voltage-dependent, N-methyl-D-aspartate (NMDA) receptor antagonists--in vitro characterization". Journal of Neural Transmission. 114 (12): 1529–37. doi:10.1007/s00702-007-0792-7. PMID 17728997.
- Danysz, W; Parsons, CG; Jirgensons, A; Kauss, V; Tillner, J (2002). "Amino-alkyl-cyclohexanes as a novel class of uncompetitive NMDA receptor antagonists". Current Pharmaceutical Design. 8 (10): 835–43. doi:10.2174/1381612024607117. PMID 11945134.
- Danysz, W; Parsons, CG (Mar 2002). "Neuroprotective potential of ionotropic glutamate receptor antagonists". Neurotoxicity Research. 4 (2): 119–26. doi:10.1080/10298420290015872. PMID 12829411.
- Clinical trial number NCT00405886 for "Neramexane for Tinnitus" at ClinicalTrials.gov
- Clinical trial number NCT00739635 for "Efficacy, Safety and Tolerability of Neramexane in Patients With Subjective Tinnitus" at ClinicalTrials.gov
- Rammes, G; Schierloh, A (Feb 2006). "Neramexane (merz pharmaceuticals/forest laboratories)". IDrugs. 9 (2): 128–35. PMID 16523403.
- Kotlinska, J; Biala, G; Rafalski, P; Bochenski, M; Danysz, W (Oct 2004). "Effect of neramexane on ethanol dependence and reinforcement". European Journal of Pharmacology. 503 (1–3): 95–8. doi:10.1016/j.ejphar.2004.09.036. PMID 15496302.
- Klein, T; Magerl, W; Hanschmann, A; Althaus, M; Treede, RD (Jan 2008). "Antihyperalgesic and analgesic properties of the N-methyl-D-aspartate (NMDA) receptor antagonist neramexane in a human surrogate model of neurogenic hyperalgesia". European Journal of Pain. 12 (1): 17–29. doi:10.1016/j.ejpain.2007.02.002. PMID 17449306.
- Kos, T; Legutko, B; Danysz, W; Samoriski, G; Popik, P (Sep 2006). "Enhancement of antidepressant-like effects but not brain-derived neurotrophic factor mRNA expression by the novel N-methyl-D-aspartate receptor antagonist neramexane in mice". Journal of Pharmacology and Experimental Therapeutics. 318 (3): 1128–36. doi:10.1124/jpet.106.103697. PMID 16740621.
- Zoladz, PR; Campbell, AM; Park, CR; Schaefer, D; Danysz, W; Diamond, DM (Oct 2006). "Enhancement of long-term spatial memory in adult rats by the noncompetitive NMDA receptor antagonists, memantine and neramexane". Pharmacology Biochemistry and Behavior. 85 (2): 298–306. doi:10.1016/j.pbb.2006.08.011. PMID 17045636.
- Plazas PV, Savino J, Kracun S, et al. (July 2007). "Inhibition of the alpha9alpha10 nicotinic cholinergic receptor by neramexane, an open channel blocker of N-methyl-D-aspartate receptors". European Journal of Pharmacology. 566 (1–3): 11–9. doi:10.1016/j.ejphar.2007.03.026. PMID 17466293.
- Suckfüll, M; Althaus, M; Ellers-Lenz, B; Gebauer, A; Görtelmeyer, R; Jastreboff, PJ; Moebius, HJ; Rosenberg, T; Russ, H; Wirth, Y; Krueger, H (Jan 2011). "A randomized, double-blind, placebo-controlled clinical trial to evaluate the efficacy and safety of neramexane in patients with moderate to severe subjective tinnitus". BMC Ear, Nose and Throat Disorders. 11: 1. doi:10.1186/1472-6815-11-1. PMC 3031239. PMID 21223542.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.