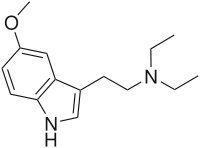
5-MeO-DET
5-MeO-DET or 5-methoxy-N,N-diethyltryptamine is a hallucinogenic tryptamine.
 | |
 | |
| Legal status | |
|---|---|
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C15H22N2O |
| Molar mass | 246.354 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Pharmacology
5-MeO-DET inhibits serotonin reuptake with an IC50 value of 2.4 μM and activates 5-HT2A receptors with an EC50 value of 8.11 nM.[1][2][3][4][5][6][7]
Effects
Low dosages (0.5–1 mg) are reported to produce a relaxing body high and mild entheogenic effects. Shulgin reports in TiHKAL that higher dosages (1–3 mg) can produce very unpleasant reactions.
See also
References
- Schulze-Alexandru M, Kovar KA, Vedani A (December 1999). "Quasi-atomistic Receptor Surrogates for the 5-HT2A Receptor: A 3D-QSAR Study on Hallucinogenic Substances". Quantitative Structure-Activity Relationships. 18 (6): 548–560. CiteSeerX 10.1.1.669.1877. doi:10.1002/(SICI)1521-3838(199912)18:6<548::AID-QSAR548>3.0.CO;2-B.
- Gatch MB, Forster MJ, Janowsky A, Eshleman AJ (July 2011). "Abuse liability profile of three substituted tryptamines". The Journal of Pharmacology and Experimental Therapeutics. 338 (1): 280–9. doi:10.1124/jpet.111.179705. PMC 3126641. PMID 21474568.
- Glennon RA, Gessner PK (April 1979). "Serotonin receptor binding affinities of tryptamine analogues". Journal of Medicinal Chemistry. 22 (4): 428–32. doi:10.1021/jm00190a014. PMID 430481.
- Halberstadt AL, Geyer MA (September 2011). "Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens". Neuropharmacology. 61 (3): 364–81. doi:10.1016/j.neuropharm.2011.01.017. PMC 3110631. PMID 21256140.
- Gessner PK, Godse DD, Krull AH, McMullan JM (March 1968). "Structure-activity relationships among 5-methoxy-n:n-dimethyltryptamine, 4-hydroxy-n:n-dimethyltryptamine (psilocin) and other substituted tryptamines". Life Sciences. 7 (5): 267–77. doi:10.1016/0024-3205(68)90200-2. PMID 5641719.
- Lyon RA, Titeler M, Seggel MR, Glennon RA (January 1988). "Indolealkylamine analogs share 5-HT2 binding characteristics with phenylalkylamine hallucinogens". European Journal of Pharmacology. 145 (3): 291–7. doi:10.1016/0014-2999(88)90432-3. PMID 3350047.
- Blough BE, Landavazo A, Decker AM, Partilla JS, Baumann MH, Rothman RB (October 2014). "Interaction of psychoactive tryptamines with biogenic amine transporters and serotonin receptor subtypes". Psychopharmacology. 231 (21): 4135–44. doi:10.1007/s00213-014-3557-7. PMC 4194234. PMID 24800892.
External links
Drugs mentioned in TiHKAL | |
|---|---|
|
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.