JWH-051
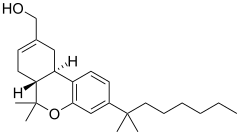
JWH-051 is an analgesic drug which is a cannabinoid agonist. Its chemical structure is closely related to that of the potent cannabinoid agonist HU-210, with the only difference being the removal of the hydroxyl group at position 1 of the aromatic ring. It was discovered and named after John W. Huffman.
 | |
| Identifiers | |
|---|---|
| |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C25H38O2 |
| Molar mass | 370.577 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
JWH-051 retains high affinity for the CB1 receptor, but is a much stronger agonist for CB2, with a Ki value of 14nM at CB2 vs 19nM at CB1.[1] It was one of the first CB2-selective ligands developed, although its selectivity for CB2 is modest compared to newer compounds such as HU-308.
It has similar effects to other cannabinoid agonists such as sedation and analgesia, but with a relatively strong antiinflammatory effect due to its strong activity at CB2.[2][3][4]
References
- Huffman JW, Yu S, Showalter V, Abood ME, Wiley JL, Compton DR, et al. (September 1996). "Synthesis and pharmacology of a very potent cannabinoid lacking a phenolic hydroxyl with high affinity for the CB2 receptor". Journal of Medicinal Chemistry. 39 (20): 3875–7. doi:10.1021/jm960394y. PMID 8831752.
- Huffman JW (September 2000). "The search for selective ligands for the CB2 receptor". Current Pharmaceutical Design. 6 (13): 1323–37. doi:10.2174/1381612003399347. PMID 10903395.
- Klein TW, Newton C, Friedman H (1998). "Cannabinoid receptors and the cytokine network". Advances in Experimental Medicine and Biology. 437: 215–22. doi:10.1007/978-1-4615-5347-2_24. ISBN 978-0-306-45838-5. PMID 9666274. Cite journal requires
|journal=(help) - Griffin G, Fernando SR, Ross RA, McKay NG, Ashford ML, Shire D, et al. (November 1997). "Evidence for the presence of CB2-like cannabinoid receptors on peripheral nerve terminals". European Journal of Pharmacology. 339 (1): 53–61. doi:10.1016/S0014-2999(97)01336-8. PMID 9450616.