Combined injectable birth control
| Combined injectable birth control | |
|---|---|
| Background | |
| Type | Hormonal |
| First use | About 1980 |
| Failure rates (first year) | |
| Perfect use | 0–0.2%[1] |
| Typical use | ? |
| Usage | |
| Duration effect | 1 month |
| User reminders | ? |
| Advantages and disadvantages | |
| STI protection | No |
| Benefits | Especially good if poor pill compliance |
Combined injectable contraceptives (CICs) are a form of hormonal birth control for women. They consist of monthly injections of combined formulations containing an estrogen and a progestin to prevent pregnancy.
CICs are different from progestogen-only injectable contraceptives (POICs), such as depot medroxyprogesterone acetate (DMPA; brand names Depo-Provera, Depo-SubQ Provera 104) and norethisterone enantate (NETE; brand name Noristerat), which are not combined with an estrogen and are given once every two to three months instead of once a month.[2]
Hormonal contraception works primarily by preventing ovulation, but it may also thicken the cervical mucus inhibiting sperm penetration.[3][4][5] Hormonal contraceptives also have effects on the endometrium,[6][7] that theoretically could affect implantation,[8][9][10][11]
Medical uses
CICs are administered by intramuscular injection into the deltoid, gluteus maximus, or anterior thigh.[1] They are ideally administered every 28 to 30 days, though they have been demonstrated to be effective up to 33 days.[1]
Some CICs have been said to be used by transgender women as a means of feminizing hormone therapy as well.[12]
Available forms
A variety of different CICs, generally containing a short-acting natural estradiol ester and a long-acting progestin ester, are available for clinical use.[13][14][2][15][16] Estrogens that are used include estradiol valerate, estradiol cypionate, estradiol enantate, estradiol benzoate butyrate, and estradiol, while progestins that are used include norethisterone enantate, medroxyprogesterone acetate, algestone acetophenide (dihydroxyprogesterone acetophenide), hydroxyprogesterone caproate, and megestrol acetate.[14][2][15][16] Estradiol benzoate has a duration that is too short for once-monthly CICs, and is not used in them.[17] Conversely, estradiol enantate is said to have a duration that is too long for once-monthly CICs, but is nonetheless used in them.[17]
| Composition | Dose | Vehicle | Brand Names | Availability |
|---|---|---|---|---|
| Estradiol valerate Norethisterone enantate | 5 mg 50 mg | Oil solution | Chinese Injectable No. 3, Efectimes, Ginediol, Mesigyna, Mesilar, Meslart, Mesocept, Mesygest, Nofertyl, Nofertyl Lafrancol, Noregyna, Norestrin, Norifam, Norigynon, Nostidyn, Sexseg, Solouna | Approved in at least 36 countries, including Argentina, the Bahamas, Barbados, Bolivia, Brazil, Chile, Colombia, Costa Rica, the Dominican Republic, Ecuador, Egypt, El Salvador, Ghana, Grenada, Guatemala, Guyana, Haiti, Honduras, Jamaica, Kenya, Mexico, Nicaragua, Panama, Paraguay, Peru, St. Lucia, Turkey, Uruguay, Venezuela, and Zimbabwe |
| Estradiol cypionate Medroxyprogesterone acetate | 5 mg 25 mg | Microcrystalline aqueous suspension | Ciclofem, Ciclofemina, Cyclofem, Cyclofemina, Cyclogeston, Femelin, Femydrol, Gestin, Harmonis, Lunella, Lunelle†, Novafem | Approved in at least 18 countries, including Bolivia, Brazil, Chile, China, Colombia, Costa Rica, El Salvador, Guatemala, Hong Kong, Indonesia, Malaysia, Mexico, Panama, Peru, Puerto Rico†, Thailand, the United States†, and Zimbabwe |
| Estradiol enantate Algestone acetophenidea | 10 mg 150 mg | Oil solution | Acefil, Agurin†, Atrimon†, Ciclomes, Ciclovar, Ciclovular, Cicnor†, Clinomin, Cycloven, Daiva, Damix, Deladroxate§, Deprans, Deproxone, Exuna, Ginestest, Ginoplan†, Gynomes, Horprotal, Listen, Luvonal, Neogestar, Neolutin, Nomagest, Nonestrol, Normagest, Normensil, Novular, Oterol, Ovoginal, Patector, Patectro, Perludil, Perlumes, Perlutal, Perlutale, Perlutan, Perlutin, Perlutin-Unifarma, Permisil, Preg-Less, Pregnolan, Progestrol†, Protegin, Proter, Seguralmes, Synovular, Topasel, Unigalen, Uno-Ciclo, Vagital | Approved in at least 19 countries, including Argentina, Belize, Brazil, Chile, Colombia, Costa Rica, the Dominican Republic, Ecuador, El Salvador, Guatemala, Honduras, Hong Kong, Mexico, Nicaragua, Panama, Paraguay, Peru, Portugal†, and Spain† |
| 5 mg 75 mg | Oil solution | Anafertin†, Patector NF, Yectames | Approved at least 9 countries, including Costa Rica, the Dominican Republic, El Salvador, Guatemala, Honduras, Mexico, Nicaragua, Panama, and Spain† | |
| 10 mg 120 mg | Oil solution | Unalmes, Yectuna | Approved in at least 3 countries, including Brazil†, Chile, and Paraguay | |
| 10 mg 75 mg | Oil solution | Ova Repos† | Discontinued | |
| Estradiol benzoate butyrate Algestone acetophenide | 10 mg 150 mg | Oil solution? | Redimen, Soluna, Unijab, Unimens§ | Approved in Peru and Singapore |
| Estradiol valerate Hydroxyprogesterone caproate | 5 mg 250 mg | Oil solution | Chinese Injectable No. 1 | Approved in China |
| Estradiol Megestrol acetate | 3.5 mg 25 mg | Microcrystalline aqueous suspension with a defined particle size range | Chinese Injectable No. 2, Mego-E | Approved in China |
| Notes: All are given by intramuscular injection once a month. Footnotes: † = Discontinued. § = Never marketed. a = Unsorted brand names (doses unknown; for E2-EN/DHPA): Evitas†, Femineo†, and Primyfar†. Miscellaneous: Direct link to table. Sources:[18][2][19][14][15][20][21][22][23][24][25] | ||||
Side effects
The most prominent side effects of CICs are menstrual irregularities during the first 3 to 6 months of use.[1]
Pharmacology
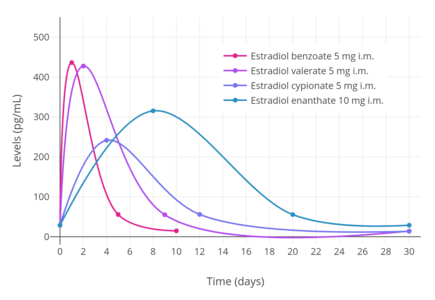
CICs contain an estrogen and a progestin. The estrogen is generally a short-acting estradiol ester, which acts as a prodrug of estradiol.[13] Esters of estradiol are natural and bioidentical estrogens, and are believed to have more favorable effects on lipid metabolism, cardiovascular health, and hemostasis than synthetic estrogens such as ethinylestradiol.[26][27][28] The progestin is a long-acting progestogen ester, which may or may not act as a prodrug.[13] Progesterone derivatives including medroxyprogesterone acetate, algestone acetophenide (dihydroxyprogesterone acetophenide), hydroxyprogesterone caproate, and megestrol acetate are active themselves and are not prodrugs, whereas the testosterone derivative norethisterone enantate is a prodrug of norethisterone. Regardless of whether they are prodrugs or not, steroid esters form a depot and have an extended duration of action due to a depot effect when administered by intramuscular or subcutaneous injection.
Because CICs are administered parenterally, they bypass the first-pass effect in the liver and intestines that occurs with oral administration of estrogens.[13] However, is estimated that about 20% of an administered dose does still eventually pass through the liver.[13] Hence, these preparations are not completely liver-neutral.[13] Nonetheless, they have dramatically reduced hepatic effects relative to oral ethinylestradiol.[29] In addition, parenteral estradiol in general has about 4- or 5-fold reduced potency in the liver than oral estradiol.[29]
History
Two CICs were introduced for clinical use by 1976: estradiol enantate/algestone acetophenide (E2-EN/DHPA; brand names Perlutan, Topasel) in Spain and Latin America, and estradiol valerate/hydroxyprogesterone caproate (EV/OHPC; brand name Injectable No. 1) in China.[30] These CICs have been described as first-generation CICs.[30] Two second-generation CICs, estradiol cypionate/medroxyprogesterone acetate (EC/MPA; brand names Cyclofem and later Lunelle) and estradiol valerate/norethisterone enantate (EV/NETE; brand name Mesigyna), were introduced for clinical use in 1993.[31][32][14]
United States
- October 5, 2000, Pharmacia received FDA approval for Lunelle Monthly Contraceptive Injection.[1]
- April 2003, Pharmacia acquired by Pfizer (makers of depot medroxyprogesterone acetate).
- October 2003, Lunelle was discontinued in the United States.
Society and culture
Availability
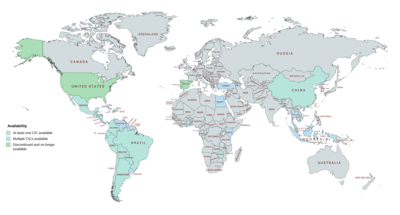
CICs are available in many countries throughout the world, including widely throughout Central and South America, in Mexico and the Caribbean, in China, in several Southeast Asian and African countries, and in Turkey.[23][24][25][16][2][32][14][15][33] They were also previously available in the United States, Portugal, and Spain, but have been discontinued in these countries.[24][25]
Research
Many other CICs have been studied but have not been approved or marketed for clinical use.[14][15][34][17][35][2]
The following are marketed CICs at different doses than those that are approved:
- Estradiol valerate 2.5 to 5 mg + norethisterone enantate 50 to 80 mg in an oil solution[14][15]
- Estradiol valerate 10 mg + hydroxyprogesterone caproate 500 mg in an oil solution[14]
- Estradiol valerate 20 mg + medroxyprogesterone acetate 100 mg in an aqueous suspension[14][15]
- Estradiol cypionate 2.5 to 10 mg + medroxyprogesterone acetate 12.5 to 50 mg in a microcrystalline aqueous suspension[14][15]
- Estradiol enantate 5 to 50 mg + algestone acetophenide 75 to 200 mg in an oil solution[34][14]
The half-progestin-dose formulation of estradiol valerate/norethisterone enantate (5 mg / 25 mg) is also known as HRP-103 and the half-progestin-dose formulation of estradiol cypionate/medroxyprogesterone acetate (5 mg / 12.5 mg) is also known as HRP-113.[36]
The following are CICs that have never been marketed:
- Estradiol undecylate 5 to 50 mg + norethisterone enantate 30 to 70 mg[17][14][35][37]
- Estradiol cypionate + norethisterone enantate[35][14]
- Estradiol valerate 10 mg + methenmadinone caproate 60 mg (Lutofollin)[17][35][37]
- Estradiol hexahydrobenzoate 5 mg + norgestrel 25 mg[17][14][35][37]
- Estradiol cypionate 3.5 to 5 mg + megestrol acetate 25 mg in a microcrystalline aqueous suspension (marketed in China?)[17][14][35]
- Estradiol valerate 3 to 5 mg + chlormadinone caproate 80 mg in an oil solution[17][14][35]
- Estradiol valerate 5 mg + megestrol acetate 15 mg in an aqueous suspension of gelatin microspheres (50–80 μm)[17][15][35][28]
- Estradiol 5 mg + levonorgestrel 7 mg in an aqueous suspension of monolithic microspheres (80 μm) or in a macrocrystalline suspension (15 μm)[15][28]
- Estradiol cypionate 5 mg + levonorgestrel butanoate 7 mg in an aqueous suspension[15]
- Mestranol 1.0–1.2 mg + norethisterone 10–12 mg in a microcrystalline aqueous suspension of defined particle sizes (125–177 μm)[17][15][35][28]
- Ethinylestradiol + norethisterone[14]
- Estradiol 5 mg and progesterone 100 to 300 mg in an aqueous suspension of monolithic microspheres or in a macrocrystalline suspension[17][15][2][14][38][39][28]
See also
References
- 1 2 3 4 5 "FDA Approves Combined Monthly Injectable Contraceptive". Contraception Report. 12 (3). 2001. Archived from the original on September 26, 2006.
- 1 2 3 4 5 6 7 Bagade O, Pawar V, Patel R, Patel B, Awasarkar V, Diwate S (2014). "Increasing use of long-acting reversible contraception: safe, reliable, and cost-effective birth control" (PDF). World J Pharm Pharm Sci. 3 (10): 364–392. ISSN 2278-4357.
- ↑ Tamara Callahan MD , Aaron Caughey MD , Blueprints Obstetrics and Gynecology, 2013
- ↑ KD Tripathi , Essentials of Medical Pharmacology, 2013
- ↑ Dc Dutta's Textbook of Obstetrics, 2014
- ↑ K. A. Petrie, A. H. Torgal, C. L. Westhoff, Matched-pairs analysis of ovarian suppressionduring oral vs. vaginal hormonal contraceptive use, „Contraception” 2011, t. 84, p. e2-3
- ↑ R. L. Birtch, O. A. Olatunbosum, R. A. Pierson, Ovarian follicular dynamics during conventional vs continuous oral contraceptive use, „Contraception” 2006, t. 73, p. 235. p. 239.
- ↑ K. Bugge, K. S. Richter, J. Bromer, et al., Pregnancy rates following in vitro fertilization are reduced with a thin endometrium, but are unrelated to endometrial thickness above 10 millimeters,„Fertility and Sterility” 2004, t. 82, p. S199.
- ↑ T. Fiumino, A. Kuwata, A. Teranischi et al., Significance of endometrium thickness to evaluate endometrial receptivity for embryos in natural cycle, „Fertility and Sterility” 2008, t. 90,p. S159.
- ↑ K. S. Richter, K. R. Bugge, J. G. Bromer, Relationship between endometrial thickness and embryo implantation, based on 1. 294 cycles of in vitro fertilization with transfer of two blastocyst-stage embryos, „Fertility and Sterility” 2007, t. 87, p. 53.
- ↑ Rivera R, Yacobson I, Grimes D (1999). "The mechanism of action of hormonal contraceptives and intrauterine contraceptive devices". Am J Obstet Gynecol. 181 (5 Pt 1): 1263–9. doi:10.1016/S0002-9378(99)70120-1. PMID 10561657.
- ↑ Don Kulick (12 January 2009). Travesti: Sex, Gender, and Culture among Brazilian Transgendered Prostitutes. University of Chicago Press. pp. 64–66. ISBN 978-0-226-46101-4.
- 1 2 3 4 5 6 V. Unzeitig; Rick H.W. van Lunsen (15 February 2000). Contraceptive Choices and Realities: Proceedings of the 5th Congress of the European Society of Contraception. CRC Press. pp. 133, 136. ISBN 978-1-85070-067-8.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Newton JR, D'arcangues C, Hall PE (1994). "A review of "once-a-month" combined injectable contraceptives". J Obstet Gynaecol (Lahore). 4 Suppl 1: S1–34. doi:10.3109/01443619409027641. PMID 12290848.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 Garza-Flores J (April 1994). "Pharmacokinetics of once-a-month injectable contraceptives". Contraception. 49 (4): 347–59. doi:10.1016/0010-7824(94)90032-9. PMID 8013219.
- 1 2 3 IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer (1 January 1999). Hormonal Contraception and Post-menopausal Hormonal Therapy (PDF). IARC. p. 65. ISBN 978-92-832-1272-0.
- 1 2 3 4 5 6 7 8 9 10 11 Toppozada MK (April 1994). "Existing once-a-month combined injectable contraceptives". Contraception. 49 (4): 293–301. doi:10.1016/0010-7824(94)90029-9. PMID 8013216.
- ↑ IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer (1 January 1999). Hormonal Contraception and Post-menopausal Hormonal Therapy (PDF). IARC. p. 65. ISBN 978-92-832-1272-0.
- ↑ Pramilla Senanayake; Malcolm Potts (14 April 2008). Atlas of Contraception, Second Edition. CRC Press. pp. 50–. ISBN 978-0-203-34732-4.
- ↑ IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer (2007). Combined Estrogen-progestogen Contraceptives and Combined Estrogen-progestogen Menopausal Therapy. World Health Organization. pp. 431–. ISBN 978-92-832-1291-1.
- ↑ Klitsch M (1995). "Still waiting for the contraceptive revolution". Fam Plann Perspect. 27 (6): 246–53. doi:10.2307/2136177. PMID 8666089.
- ↑ Gallo MF, Grimes DA, Lopez LM, Schulz KF, d'Arcangues C (2013). "Combination injectable contraceptives for contraception". Cochrane Database Syst Rev. 3: CD004568. doi:10.1002/14651858.CD004568.pub3. PMID 23641480.
- 1 2 https://www.drugs.com/international/
- 1 2 3 Sweetman, Sean C., ed. (2009). "Sex hormones and their modulators". Martindale: The Complete Drug Reference (36th ed.). London: Pharmaceutical Press. p. 2082. ISBN 978-0-85369-840-1.
- 1 2 3 http://www.micromedexsolutions.com/micromedex2/librarian/
- ↑ Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. pp. 235–237, 261, 271. ISBN 978-3-642-60107-1.
Natural estrogens considered here include: [...] Esters of 17β-estradiol, such as estradiol valerate, estradiol benzoate and estradiol cypionate. Esterification aims at either better absorption after oral administration or a sustained release from the depot after intramuscular administration. During absorption, the esters are cleaved by endogenous esterases and the pharmacologically active 17β-estradiol is released; therefore, the esters are considered as natural estrogens.
- ↑ Nagrath Arun; Malhotra Narendra; Seth Shikha (15 December 2012). Progress in Obstetrics and Gynecology--3. Jaypee Brothers Medical Publishers Pvt. Ltd. pp. 419–. ISBN 978-93-5090-575-3.
- 1 2 3 4 5 Sang GW (April 1994). "Pharmacodynamic effects of once-a-month combined injectable contraceptives". Contraception. 49 (4): 361–85. doi:10.1016/0010-7824(94)90033-7. PMID 8013220.
- 1 2 Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- 1 2 J. Bringer; B. Hedon (15 September 1995). Fertility and Sterility: A Current Overview. CRC Press. pp. 47–. ISBN 978-1-85070-694-6.
- ↑ d'Arcangues C (1993). "Once-a-month injectable contraceptives". World Health Forum. 14 (4): 439–40. PMID 8185807.
- 1 2 Pramilla Senanayake; Malcolm Potts (14 April 2008). Atlas of Contraception, Second Edition. CRC Press. pp. 50–. ISBN 978-0-203-34732-4.
- ↑ IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer (2007). Combined Estrogen-progestogen Contraceptives and Combined Estrogen-progestogen Menopausal Therapy. World Health Organization. pp. 431–. ISBN 978-92-832-1291-1.
- 1 2 Koetsawang S (April 1994). "Once-a-month injectable contraceptives: efficacy and reasons for discontinuation". Contraception. 49 (4): 387–98. doi:10.1016/0010-7824(94)90034-5. PMID 8013221.
- 1 2 3 4 5 6 7 8 9 Goldsmith, A., & Toppozada, M. (1983). Long-acting contraception. pp. 94-95 https://www.popline.org/node/423289
- ↑ Unlisted Drugs Pharm AID. Unlisted Drugs. 1993. p. 247. ISBN 978-0-913210-14-7.
- 1 2 3 Toppozada M (June 1977). "The clinical use of monthly injectable contraceptive preparations". Obstet Gynecol Surv. 32 (6): 335–47. doi:10.1097/00006254-197706000-00001. PMID 865726.
- ↑ Garza-Flores J, Fatinikun T, Hernandez L, Ramos I, Cardenas M, Menjivar M (July 1991). "A pilot study on the assessment of a progesterone/estradiol sustained release as once-a-month-injectable contraceptive". Contraception. 44 (1): 45–59. doi:10.1016/0010-7824(91)90105-O. PMID 1893701.
- ↑ Garza-Flores J, Hall PE, Perez-Palacios G (1991). "Long-acting hormonal contraceptives for women". J. Steroid Biochem. Mol. Biol. 40 (4–6): 697–704. doi:10.1016/0960-0760(91)90293-E. PMID 1958567.
Further reading
- Garza-Flores J (April 1994). "Pharmacokinetics of once-a-month injectable contraceptives". Contraception. 49 (4): 347–59. doi:10.1016/0010-7824(94)90032-9. PMID 8013219.
- Sang GW (April 1994). "Pharmacodynamic effects of once-a-month combined injectable contraceptives". Contraception. 49 (4): 361–85. doi:10.1016/0010-7824(94)90033-7. PMID 8013220.