Estradiol valerate
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation |
/ˌɛstrəˈdaɪoʊl ES-trə-DY-ohl VAL-ə-rayt[1] |
| Trade names | Delestrogen, Progynon Depot, Progynova, many others |
| Synonyms | EV; E2V; Oestradiol valerate; Estradiol pentanoate; Estradiol valerianate |
| Routes of administration | By mouth, intramuscular injection,[2] subcutaneous injection |
| Drug class | Estrogen; Estrogen ester |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability |
Oral: 3–5%[3][4] IM: 100%[3] |
| Metabolism | Cleavage via esterases in the liver, blood, and tissues[3] |
| Metabolites | Estradiol, valeric acid, and metabolites of estradiol[3] |
| Elimination half-life |
Oral: 12–20 hours (as E2)[3][5] IM: 4–5 days[3] |
| Duration of action |
IM (5 mg): 7–8 days[6] IM (10–30 mg): 1–4 weeks[7] |
| Excretion | Urine (80%)[3] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard |
100.012.327 |
| Chemical and physical data | |
| Formula | C23H32O3 |
| Molar mass | 356.498 g/mol |
| 3D model (JSmol) | |
| |
| |
Estradiol valerate, sold under the brand names Delestrogen, Progynon Depot, and Progynova among others, is an estrogen medication which is used in hormone therapy for menopausal symptoms and low estrogen levels in women, in hormone therapy for transgender women, and in hormonal birth control for women.[4][3][7][8] It is also used in the treatment of prostate cancer in men.[7] The medication is taken by mouth or by injection into muscle once every 1 to 4 weeks.[7][8]
Side effects of estradiol valerate include breast tenderness, breast enlargement, nausea, headache, and fluid retention.[9][7][8] Estradiol valerate is a synthetic estrogen and hence is an agonist of the estrogen receptor (ER), the biological target of estrogens like estradiol.[4][3][10] It is an estrogen ester and a prodrug of estradiol in the body.[10][4][3] Because of this, it is considered to be a natural and bioidentical form of estrogen.[10][3][11]
Estradiol valerate was first described in 1940 and was introduced for medical use in 1954.[12][13][14] Along with estradiol cypionate, it is one of the most widely used esters of estradiol.[15] Estradiol valerate is used in the United States, Canada, Europe, and throughout much of the rest of the world.[16][17] It is available as a generic medication.[18]
Medical uses
The medical uses of estradiol valerate are the same as those of estradiol and other estrogens. Examples of indications for the medication include hormone therapy and hormonal contraception. In regard to the latter, estradiol valerate is available in combination with a progestin as an combined estradiol-containing oral contraceptive (with dienogest)[19] and as a combined injectable contraceptive.[20][21][22] Along with estradiol cypionate, estradiol undecylate, and estradiol benzoate, estradiol valerate is used as a form of high-dose estrogen therapy in feminizing hormone therapy for transgender women.[23][24][25][26] It is also used as a form of high-dose estrogen therapy in the treatment of prostate cancer in men.[7]
In the United States, the approved indications of estradiol valerate injections include the treatment of moderate to severe hot flashes and vaginal atrophy associated with menopause in women, the treatment of hypoestrogenism due to hypogonadism, castration, or primary ovarian failure in women, and the palliative treatment of advanced prostate cancer in men.[7] Elsewhere in the world, oral estradiol valerate is similarly approved for the treatment of symptoms associated with menopause or hypoestrogenism due to castration in women.[8] Such symptoms may include hot flashes, outbreaks of sweat, sleep disturbances, depressive moods, irritability, headaches, and dizziness.[8]
Estradiol valerate by intramuscular injection is usually used at a dosage of 10 to 20 mg every 4 weeks in the treatment of menopausal symptoms and hypoestrogenism due to hypogonadism, castration, or primary ovarian failure in women.[7] It is usually used in the treatment of advanced prostate cancer in men at a dosage of 30 mg or more every 1 to 2 weeks by intramuscular injection.[7] In transgender women, estradiol valerate given by intramuscular injection is usually used at a dosage of 5 to 20 mg, but up to 30 to 40 mg, once every 2 weeks.[24][25][23]
Available forms
Estradiol valerate is and has been available in the form of vials and ampoules for intramuscular injection in concentrations of 5, 10, 20, and 40 mg/mL and in the form of oral tablets at doses of 0.5, 1, 2, and 4 mg per tablet.[27][12][28][29] In the United States, it is specifically available in formulations of 10, 20, and 40 mg/mL (as Delestrogen, as well as generics).[27] Aside from estradiol valerate, the only other injectable estrogen formulations that remain available in the United States are estradiol cypionate (5 mg/mL in oil) and conjugated estrogens (25 mg/vial in solution).[27]
In addition to single-drug formulations, oral estradiol valerate is available in combination with the progestin dienogest as a combined oral contraceptive and intramuscular estradiol valerate is marketed in combination with the progestins hydroxyprogesterone caproate and norethisterone enantate as combined injectable contraceptives.[27][19][20][21][22][1] Intramuscular estradiol valerate has also been marketed in combination with testosterone enantate, but this formulation has been discontinued.[27] The availability of estradiol valerate-containing products varies throughout the world.[1]
Side effects
The side effects of estradiol valerate are the same as those of estradiol. Examples of such side effects include breast tenderness and enlargement, nausea, bloating, edema, headache, and melasma.[9][30] High-dose estrogen therapy with estradiol valerate injections may also cause an increased risk of thromboembolism, changes in blood lipid profile, increased insulin resistance, and increased levels of prolactin.[30]
Pharmacology
Pharmacodynamics
Estradiol valerate is an estradiol ester, or a prodrug of estradiol.[10][4] As such, it is an estrogen, or an agonist of the estrogen receptors.[4][10] The affinity of estradiol valerate for the estrogen receptor is approximately 50 times lower than that of estradiol.[3] In addition, estradiol valerate is rapidly cleaved into estradiol and is unable to reach target tissues in concentrations of significance, if at all.[3] As such, estradiol valerate is essentially inactive in terms of estrogenic effect itself, acting solely as a prodrug to estradiol.[3] Estradiol valerate is of about 31% higher molecular weight than estradiol due to the presence of its C17β valerate ester.[16][17] Aside from dose adjustment to account for the difference in molecular weight, oral estradiol valerate is considered to be equivalent to oral micronized estradiol.[3] Because estradiol valerate is a prodrug of estradiol, it is considered to be a natural and bioidentical form of estrogen.[10][11]
| Estrogen | Type | EPD (mg/14 days) | EPD (mg/day) | MSD (mg/14 days) | MSD (mg/day) |
|---|---|---|---|---|---|
| Estradiol (micronized) | Bioidentical | 60 | 4.3 | 14–28 | 1.0–2.0 |
| Estradiol valerate | Bioidentical | 60 | 4.3 | 14–28 | 1.0–2.0 |
| Estriol | Bioidentical | 140–150a | 10.0–10.7a | 28–84 | 2.0–6.0 |
| Estriol succinate | Bioidentical | 140–150a | 10.0–10.7a | 28–84 | 2.0–6.0 |
| Conjugated estrogens | Natural | 60 | 4.3 | 8.4–17.5 | 0.6–1.25 |
| Ethinylestradiol | Synthetic | 1.0–1.5 | 0.071–0.11 | 0.28 | 0.02 |
| Mestranol | Synthetic | 1.5–1.8 | 0.11–0.13 | 0.35 | 0.025 |
| Quinestrol | Synthetic | 2.0–4.0 | 0.14–0.29 | ND | ND |
| Diethylstilbestrol | Synthetic | 20–30 | 1.4–2.1 | ND | ND |
| Diethylstilbestrol dipropionate | Synthetic | 15–20 | 1.1–1.4 | ND | ND |
| Dienestrol diacetate | Synthetic | 40–60 | 2.9–4.3 | ND | ND |
| Addendum: The ovulation-inhibiting dose (OID) of ethinylestradiol is 0.1 mg/day.[31] Footnotes: a = Taken in divided doses three times per day. Abbreviations: EPD = Endometrial proliferation dose. MSD = Menopausal substitution dose. Miscellaneous: Direct link to table. Sources: [32][33][34][35] | |||||
| Estrogen | Type | EPD (14 days) | Duration | |
|---|---|---|---|---|
| Estradiol benzoate | Bioidentical | 25–30 mg | 5 mg ≈ 5 days | |
| Estradiol dipropionate | Bioidentical | 25–30 mg | 5 mg ≈ 5–8 days | |
| Estradiol valerate | Bioidentical | 20 mg | 10 mg ≈ 14 days | |
| Estradiol cypionate | Bioidentical | 25–30 mg | 5 mg ≈ 14 days | |
| Polyestradiol phosphate | Bioidentical | 40–60 mg | 40 mg ≈ 28 days | |
| Diethylstilbestrol | Synthetic | 20 mg | 3 mg ≈ 3 days | |
| Diethylstilbestrol dipropionate | Synthetic | 15 mg | 2.5 mg ≈ 5 days | |
| Addendum: An effective ovulation-inhibiting dose of estradiol undecylate is 20–30 mg/month.[36] Notes: All of the estrogens are by intramuscular injection. Abbreviations: EPD = Endometrial proliferation dose. Miscellaneous: Direct link to table. Sources: [37][38] | ||||
Pharmacokinetics
Regardless of the route of administration, estradiol valerate behaves as a prodrug of estradiol via cleavage by esterases into estradiol and the natural fatty acid valeric acid.[4][10][3][4][39] This cleavage occurs not only in the liver, but also in the blood and in tissues, and the hydrolysis of estradiol valerate into estradiol and valeric acid is complete regardless of whether the drug is administered orally or parenterally.[3] High levels of circulating estradiol are found after an intravenous injection of estradiol valerate, and this indicates very rapid cleavage of the drug upon entering circulation.[3] In contrast to estradiol, which can distribute into and exert its effects in target tissues, valeric acid is quickly metabolized via beta oxidation (see also fatty acid metabolism).[3]
Oral administration
The esterification of the C17β position of estradiol as in estradiol valerate prevents the metabolism of estradiol valerate by 17β-hydroxysteroid dehydrogenase (17β-HSD).[4] As approximately 80% of estradiol is metabolized into estrone (and estrone sulfate) by 17β-HSD during first-pass metabolism, this improves the metabolic stability and hence bioavailability of estradiol valerate.[10] However, estradiol valerate is hydrolyzed into estradiol and valeric acid in the intestines, and hence, is still subject to extensive (albeit comparatively reduced) first-pass metabolism.[4] As such, the oral bioavailability of estradiol valerate is only around 3 to 5%, and is similar to that of micronized estradiol (which has similarly improved bioavailability relative to (non-micronized) oral estradiol).[3][4][40] Due to its nature as a rapidly converted prodrug of estradiol, the pharmacokinetics of estradiol valerate are similar to those of micronized estradiol.[3][4] Moreover, the pharmacodynamics and potency (after differences in molecular weight are taken into account) of oral estradiol valerate are considered to be equivalent to those of micronized estradiol.[3] This is also notably true for effects on hepatic protein synthesis (e.g., of SHBG), again after differences in molecular weight between the two drugs are considered.[3]
A dosage of 1 mg/day oral estradiol valerate has been found to produce approximate circulating concentrations of 50 pg/mL estradiol and 160 pg/mL estrone, while a dosage of 2 mg/day results in circulating levels of 60 pg/mL estradiol and 300 pg/mL estrone.[41] These concentrations of estradiol and estrone are comparable to those observed with 1 and 2 mg/day oral micronized estradiol.[41] A review of selected studies reported a range of mean peak estradiol levels of 24 to 140 pg/mL occurring 1 to 12 hours after administration of 2 mg oral estradiol valerate.[3] A study of high-dose oral estradiol valerate found levels of about 250 pg/mL after a single 10-mg dose in women.[40] This study found nearly equal concentration–time curves for 10 mg oral estradiol valerate and 10 mg oral micronized estradiol, indicating that there are no major differences between the potencies of the two forms of oral estradiol.[40] However, one study found that, in accordance with their differences in molecular weights, oral micronized estradiol produced higher levels of estradiol than oral estradiol valerate.[42]
Intramuscular injection
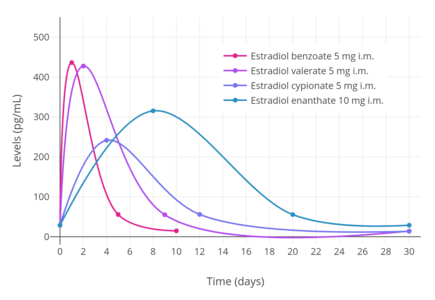
In contrast to oral administration, the bioavailability of estradiol valerate has been found to be complete (i.e., 100%) via intramuscular injection.[3][4] Due to the far greater bioavailability of intramuscular estradiol valerate relative to oral, the former is substantially stronger (in terms of potency) than the latter.[3] As an example, a single 4 mg intramuscular injection is said to be approximately equivalent to 2 mg/day of the drug administered orally over the course of 3 weeks.[3] Estradiol valerate, when given intramuscularly in oil, has a relatively long duration due to the formation of an intramuscular depot from which the drug is slowly released and absorbed.[3][44] Upon intramuscular injection of estradiol valerate in an oil solution, the solvent (i.e., oil) is absorbed, and a primary microcrystalline depot is formed within the muscle at the site of injection.[4] In addition, a secondary depot may also be formed in adipose tissue.[4] The slow release of estradiol valerate is caused by the increased lipophilicity of the drug, which in turn is due to its long fatty acid valeric acid ester moiety.[3] The terminal half-life of intramuscularly administered estradiol valerate in oil is reported to be 4 to 5 days.[3]
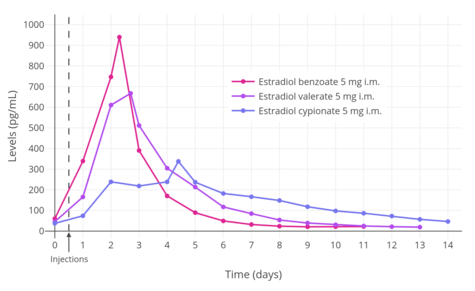
A single intramuscular injection of 4 mg estradiol valerate has been found to result in maximal circulating levels of estradiol of about 390 pg/mL within 3 days of administration, with levels declining to 100 pg/mL (baseline, in the study) by 12 to 13 days.[45] Another study found that a single intramuscular injection of 5 mg estradiol valerate resulted in peak circulating levels of 667 pg/mL estradiol and 324 pg/mL estrone within approximately 2 and 3 days, respectively (see right/above table).[6] The duration of estradiol valerate at this dose and in this study was considered to be 7 to 8 days.[6] Other studies have found that larger doses of intramuscular estradiol valerate exceeding 20 mg have a duration of more than 15 days.[6] A third study, in contrast to the preceding study, found that a single 10 mg intramuscular injection of estradiol valerate resulted in maximal estradiol levels of 506 to 544 pg/mL and maximal estrone levels of 205 to 219 pg/mL in postmenopausal women.[46]
A study of high-dose combined intramuscular administration of 40 mg estradiol valerate and 250 mg hydroxyprogesterone caproate per week for 6 months (described as a "pseudopregnancy" regimen) in hypogonadal women found that circulating levels of estradiol increased from 27.8–34.8 pg/mL to 3028–3226 pg/mL after three months and to 2491–2552 pg/mL after 6 months of treatment.[47]
| Estrogen | Peak levels | Time to peak | Duration |
|---|---|---|---|
| Estradiol cypionate | E2: 338 pg/mL E1: 145 pg/mL | E2: 3.9 days E1: 5.1 days | 11 days |
| Estradiol valerate | E2: 667 pg/mL E1: 324 pg/mL | E2: 2.2 days E1: 2.7 days | 7–8 days |
| Estradiol benzoate | E2: 940 pg/mL E1: 343 pg/mL | E2: 1.8 days E1: 2.4 days | 4–5 days |
Chemistry
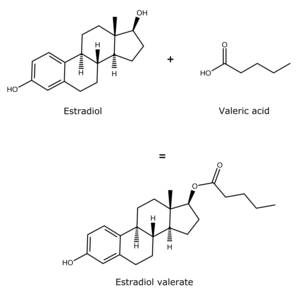
Estradiol valerate is a synthetic estrane steroid and the C17β valerate (pentanoate) fatty acid ester of estradiol.[16][17] It is also known as estradiol 17β-valerate or as estra-1,3,5(10)-triene-3,17β-diol 17β-pentanoate.[16][17] Other common esters of estradiol in use include estradiol cypionate, estradiol enantate, and estradiol acetate, the former two of which are C17β esters of estradiol similarly to estradiol valerate and the latter of which is the C3 acetate ester of estradiol.[16][17]
History
Estradiol valerate was patented by Ciba in 1940 and 1941, with a priority date of 1936.[12][48] It was first introduced for medical use by Squibb in 1954 under the brand name Delestrogen in the United States.[13][14] Subsequently, estradiol valerate was marketed widely in Europe as Progynon Depot and Progynova.[17][13] Along with estradiol benzoate (1936)[49][50] and estradiol cypionate (1952),[51] estradiol valerate has become one of the most widely used esters of estradiol.[15]
Society and culture
Generic names
Estradiol valerate is the generic name of the drug and its INN, USAN, BANM, and JAN, while oestradiol valerate was formerly its BANM.[16][17][52]
Brand names
Estradiol valerate is or has been marketed under the brand names Altadiol, Deladiol, Delestrogen, Estraval, Neofollin, Progynon Depot, Progynova, and Valergen, among many others.[16][17][52]
Availability
Oral estradiol valerate is used primarily in Europe, under the brand name Progynova.[53] Although oral estradiol valerate was previously available in the United States,[17] it is no longer available in this country except in combination with dienogest as a combined oral contraceptive (under the brand name Natazia).[27] Estradiol valerate by intramuscular injection is available under the brand name Delestrogen in the United States and Canada and under the brand name Progynon Depot in Europe and elsewhere in the world.[27][17]
See also
References
- 1 2 3 https://www.drugs.com/cons/estradiol-and-dienogest.html
- ↑ Christoph Zink (1 January 1988). Dictionary of Obstetrics and Gynecology. Walter de Gruyter. p. 86. ISBN 978-3-11-085727-6. Retrieved 20 May 2012.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 Düsterberg B, Nishino Y (December 1982). "Pharmacokinetic and pharmacological features of oestradiol valerate". Maturitas. 4 (4): 315–24. doi:10.1016/0378-5122(82)90064-0. PMID 7169965.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- ↑ Stanczyk, Frank Z.; Archer, David F.; Bhavnani, Bhagu R. (2013). "Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment". Contraception. 87 (6): 706–727. doi:10.1016/j.contraception.2012.12.011. ISSN 0010-7824. PMID 23375353.
- 1 2 3 4 5 6 7 Oriowo MA, Landgren BM, Stenström B, Diczfalusy E (April 1980). "A comparison of the pharmacokinetic properties of three estradiol esters". Contraception. 21 (4): 415–24. doi:10.1016/s0010-7824(80)80018-7. PMID 7389356.
- 1 2 3 4 5 6 7 8 9 https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/009402s052lbl.pdf
- 1 2 3 4 5
- 1 2 Amit K. Ghosh (23 September 2010). Mayo Clinic Internal Medicine Board Review. OUP USA. pp. 222–. ISBN 978-0-19-975569-1.
- 1 2 3 4 5 6 7 8 Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. p. 261. ISBN 978-3-642-60107-1.
Natural estrogens considered here include: [...] Esters of 17β-estradiol, such as estradiol valerate, estradiol benzoate and estradiol cypionate. Esterification aims at either better absorption after oral administration or a sustained release from the depot after intramuscular administration. During absorption, the esters are cleaved by endogenous esterases and the pharmacologically active 17β-estradiol is released; therefore, the esters are considered as natural estrogens.
- 1 2 Nagrath Arun; Malhotra Narendra; Seth Shikha (15 December 2012). Progress in Obstetrics and Gynecology--3. Jaypee Brothers Medical Publishers Pvt. Ltd. pp. 419–. ISBN 978-93-5090-575-3.
- 1 2 3 A. Kleemann; J. Engel; B. Kutscher; D. Reichert (14 May 2014). Pharmaceutical Substances, 5th Edition, 2009: Syntheses, Patents and Applications of the most relevant APIs. Thieme. pp. 1167–1174. ISBN 978-3-13-179525-0.
- 1 2 3 William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 1477–. ISBN 978-0-8155-1856-3.
- 1 2 Larry L. Duetsch (1969). Research and development, market power, and patent policy in ethical drugs. University of Wisconsin--Madison. p. 95.
- 1 2 Samuel S. C. Yen (1991). Reproductive endocrinology: physiology, pathophysiology, and clinical management. Saunders. ISBN 978-0-7216-3206-3. Retrieved 20 May 2012.
- 1 2 3 4 5 6 7 J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 898–. ISBN 978-1-4757-2085-3.
- 1 2 3 4 5 6 7 8 9 10 Index Nominum 2000: International Drug Directory. Taylor & Francis US. 2000. p. 405. ISBN 978-3-88763-075-1. Retrieved 20 May 2012.
- ↑ https://www.drugs.com/availability/generic-delestrogen.html
- 1 2 Guida M, Bifulco G, Di Spiezio Sardo A, Scala M, Fernandez LM, Nappi C (2010). "Review of the safety, efficacy and patient acceptability of the combined dienogest/estradiol valerate contraceptive pill". International Journal of Women's Health. 2: 279–90. doi:10.2147/IJWH.S6954. PMC 2990895. PMID 21151673.
- 1 2 Newton JR, D'arcangues C, Hall PE (1994). "A review of "once-a-month" combined injectable contraceptives". J Obstet Gynaecol (Lahore). 4 Suppl 1: S1–34. doi:10.3109/01443619409027641. PMID 12290848.
- 1 2 http://www.wjpps.com/download/article/1412071798.pdf
- 1 2 Rowlands, S (2009). "New technologies in contraception". BJOG: An International Journal of Obstetrics & Gynaecology. 116 (2): 230–239. doi:10.1111/j.1471-0528.2008.01985.x. ISSN 1470-0328.
- 1 2 Wesp LM, Deutsch MB (March 2017). "Hormonal and Surgical Treatment Options for Transgender Women and Transfeminine Spectrum Persons". Psychiatr. Clin. North Am. 40 (1): 99–111. doi:10.1016/j.psc.2016.10.006. PMID 28159148.
- 1 2 Smith KP, Madison CM, Milne NM (December 2014). "Gonadal suppressive and cross-sex hormone therapy for gender dysphoria in adolescents and adults". Pharmacotherapy. 34 (12): 1282–97. doi:10.1002/phar.1487. PMID 25220381.
- 1 2 Randi Ettner; Stan Monstrey; Eli Coleman (20 May 2016). Principles of Transgender Medicine and Surgery. Routledge. pp. 216–. ISBN 978-1-317-51460-2.
- ↑ Gianna E. Israel; Donald E. Tarver; Joy Diane Shaffer (1 March 2001). Transgender Care: Recommended Guidelines, Practical Information, and Personal Accounts. Temple University Press. pp. 64–. ISBN 978-1-56639-852-7.
- 1 2 3 4 5 6 7 "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Retrieved 16 November 2016.
- ↑ Muller (19 June 1998). European Drug Index: European Drug Registrations, Fourth Edition. CRC Press. pp. 276, 313, 379, 561, 566. ISBN 978-3-7692-2114-5.
- ↑ Kenneth L. Becker (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 2153–. ISBN 978-0-7817-1750-2.
- 1 2 Bishop BM (December 2015). "Pharmacotherapy Considerations in the Management of Transgender Patients: A Brief Review". Pharmacotherapy. 35 (12): 1130–9. doi:10.1002/phar.1668. PMID 26684553.
- ↑ N. Rietbrock; A.H. Staib; D. Loew (11 March 2013). Klinische Pharmakologie: Arzneitherapie. Springer-Verlag. pp. 426–. ISBN 978-3-642-57636-2.
- ↑ Lauritzen C (September 1990). "Clinical use of oestrogens and progestogens". Maturitas. 12 (3): 199–214. doi:10.1016/0378-5122(90)90004-P. PMID 2215269.
- ↑ Alfred S. Wolf; H.P.G. Schneider (12 March 2013). Östrogene in Diagnostik und Therapie. Springer-Verlag. pp. 78–. ISBN 978-3-642-75101-1.
- ↑ Gunther Göretzlehner; Christian Lauritzen; Thomas Römer; Winfried Rossmanith (1 January 2012). Praktische Hormontherapie in der Gynäkologie. Walter de Gruyter. pp. 44–. ISBN 978-3-11-024568-4.
- ↑ Karl Knörr; Fritz K. Beller; Christian Lauritzen (17 April 2013). Lehrbuch der Gynäkologie. Springer-Verlag. pp. 212–213. ISBN 978-3-662-00942-0.
- ↑ Toppozada M (June 1977). "The clinical use of monthly injectable contraceptive preparations". Obstet Gynecol Surv. 32 (6): 335–47. doi:10.1097/00006254-197706000-00001. PMID 865726.
- ↑ Karl Knörr; Henriette Knörr-Gärtner; Fritz K. Beller; Christian Lauritzen (8 March 2013). Lehrbuch der Geburtshilfe und Gynäkologie: Physiologie und Pathologie der Reproduktion. Springer-Verlag. pp. 508–. ISBN 978-3-662-00526-2.
- ↑ Karl Knörr; Fritz K. Beller; Christian Lauritzen (17 April 2013). Lehrbuch der Gynäkologie. Springer-Verlag. pp. 212–213. ISBN 978-3-662-00942-0.
- ↑ "Progynova 1mg (SPC) | Drugs.com". Retrieved 2012-09-06.
- 1 2 3 4 Shellenberger, T. E. (1986). "Pharmacology of estrogens": 393–410. doi:10.1007/978-94-009-4145-8_36.
- 1 2 O'Connell MB (1995). "Pharmacokinetic and pharmacologic variation between different estrogen products". J Clin Pharmacol. 35 (9 Suppl): 18S–24S. doi:10.1002/j.1552-4604.1995.tb04143.x. PMID 8530713.
- ↑ Wiegratz I, Fink T, Rohr UD, Lang E, Leukel P, Kuhl H (September 2001). "[Cross-over comparison of the pharmacokinetics of estradiol during hormone replacement therapy with estradiol valerate or micronized estradiol]". Zentralbl Gynakol (in German). 123 (9): 505–12. doi:10.1055/s-2001-18223. PMID 11709743.
- 1 2 3 4 Garza-Flores J (April 1994). "Pharmacokinetics of once-a-month injectable contraceptives". Contraception. 49 (4): 347–59. doi:10.1016/0010-7824(94)90032-9. PMID 8013219.
- ↑ Sriram. Medicinal Chemistry. Pearson Education India. p. 427. ISBN 978-81-317-0031-0. Retrieved 20 May 2012.
- ↑ M. Notelovitz; P.A. van Keep (6 December 2012). The Climacteric in Perspective: Proceedings of the Fourth International Congress on the Menopause, held at Lake Buena Vista, Florida, October 28–November 2, 1984. Springer Science & Business Media. pp. 399–. ISBN 978-94-009-4145-8.
- ↑ Schug BS, Donath F, Blume HH (2012). "Bioavailability and pharmacodynamics of two 10-mg estradiol valerate depot formulations following IM single dose administration in healthy postmenopausal volunteers". Int J Clin Pharmacol Ther. 50 (2): 100–17. doi:10.5414/cp201589. PMID 22257576.
- ↑ Ulrich U, Pfeifer T, Lauritzen C (1994). "Rapid increase in lumbar spine bone density in osteopenic women by high-dose intramuscular estrogen-progestogen injections. A preliminary report". Horm. Metab. Res. 26 (9): 428–31. doi:10.1055/s-2007-1001723. PMID 7835827.
- ↑ https://patents.google.com/patent/US2205627A/en
- ↑ Enrique Raviña; Hugo Kubinyi (16 May 2011). The Evolution of Drug Discovery: From Traditional Medicines to Modern Drugs. John Wiley & Sons. p. 175. ISBN 978-3-527-32669-3. Retrieved 20 May 2012.
- ↑ Folley SJ (December 1936). "The effect of oestrogenic hormones on lactation and on the phosphatase of the blood and milk of the lactating cow" (PDF). The Biochemical Journal. 30 (12): 2262–72. PMC 1263335. PMID 16746289.
- ↑ Marshall Sittig (1 January 1988). Pharmaceutical Manufacturing Encyclopedia. William Andrew. pp. 575–576. ISBN 978-0-8155-1144-1. Retrieved 20 May 2012.
- 1 2 https://www.drugs.com/international/estradiol.html
- ↑ Joseph S. Sanfilippo (January 1998). Primary Care in Obstetrics and Gynecology: A Handbook for Clinicians. Springer Science & Business Media. pp. 227–. ISBN 978-0-387-94739-6.
Further reading
- Vermeulen A (1975). "Longacting steroid preparations". Acta Clin Belg. 30 (1): 48–55. doi:10.1080/17843286.1975.11716973. PMID 1231448.
- Düsterberg B, Nishino Y (1982). "Pharmacokinetic and pharmacological features of oestradiol valerate". Maturitas. 4 (4): 315–24. doi:10.1016/0378-5122(82)90064-0. PMID 7169965.
- Sang GW (1994). "Pharmacodynamic effects of once-a-month combined injectable contraceptives". Contraception. 49 (4): 361–85. doi:10.1016/0010-7824(94)90033-7. PMID 8013220.