Robalzotan
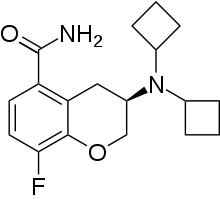
Robalzotan (NAD-299, AZD-7371) is a selective antagonist at the 5-HT1A receptor.[1] It was shown to completely reverse the autoreceptor-mediated inhibition of serotonin release induced by the administration of selective serotonin reuptake inhibitors like citalopram in rodent studies.[2] It was subsequently investigated by AstraZeneca as a potential antidepressant but like many other 5-HT1A ligands was discontinued.[3] Later on it was researched for other indications such as irritable bowel syndrome but was dropped once again.[4]
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider |
|
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C18H23FN2O2 |
| Molar mass | 318.392 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
References
- Jerning E, Svantesson GT, Mohell N (1998). "Receptor binding characteristics of [3H]NAD-299, a new selective 5-HT1A receptor antagonist". Eur J Pharmacol. 360 (2–3): 219–225. doi:10.1016/S0014-2999(98)00667-0. PMID 9851589.
- Arborelius L, Wallsten C, Ahlenius S, Svensson TH (1999). "The 5-HT(1A) receptor antagonist robalzotan completely reverses citalopram-induced inhibition of serotonergic cell firing". Eur J Pharmacol. 382 (2): 133–138. doi:10.1016/S0014-2999(99)00592-0. PMID 10528148.
- Mucke HA. (2000). "Robalzotan AstraZeneca". Curr Opin Investig Drugs. 1 (2): 236–240. PMID 11249580.
- Drossman DA, Danilewitz M, Naesdal J, Hwang C, Adler J, Silberg DG (October 2008). "Randomized, double-blind, placebo-controlled trial of the 5-HT1A receptor antagonist AZD7371 tartrate monohydrate (robalzotan tartrate monohydrate) in patients with irritable bowel syndrome". The American Journal of Gastroenterology. 103 (10): 2562–9. PMID 18775020.
| 5-HT1AR agonists | |
|---|---|
| GABAAR PAMs |
|
| Gabapentinoids (α2δ VDCC blockers) | |
| Antidepressants |
|
| Sympatholytics (Antiadrenergics) |
|
| Others | |
| |
Drugs for functional gastrointestinal disorders (A03) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drugs for functional bowel disorders |
| ||||||||||||
| Belladonna and derivatives (antimuscarinics) |
| ||||||||||||
| Propulsives | |||||||||||||
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.