D-DOPA
d-DOPA (d-3,4-dihydroxyphenylalanine; dextrodopa) is similar to l-DOPA (levodopa), but with opposite chirality. Levo- and dextro- rotation refer to a molecule's ability to rotate planes of polarized light in either direction. Whereas l-DOPA is moderately effective in the treatment of Parkinson's disease (PD) and Dopamine-responsive dystonia (DRD) by stimulating the production of dopamine in the brain, d-DOPA is biologically inactive.
 | |
| Names | |
|---|---|
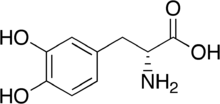
| IUPAC name
(R)-2-Amino-3-(3,4-dihydroxyphenyl)propanoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.024.858 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H11NO4 | |
| Molar mass | 197.19 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
See also
- l-DOPA (Levodopa; Sinemet, Parcopa, Atamet, Stalevo, Madopar, Prolopa, etc.)
- l-DOPS (Droxidopa)
- Methyldopa (Aldomet, Apo-Methyldopa, Dopamet, Novomedopa, etc.)
- Dopamine (Intropan, Inovan, Revivan, Rivimine, Dopastat, Dynatra, etc.)
- Norepinephrine (Noradrenaline; Levophed, etc.)
- Epinephrine (Adrenaline; Adrenalin, EpiPen, Twinject, etc.)
References
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.