meta-Tyramine
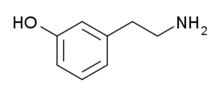
meta-Tyramine, also known as m-tyramine and 3-tyramine, is an endogenous trace amine neuromodulator and a structural analog of phenethylamine.[1][2][3] It is a positional isomer of para-tyramine, and similarly to it, has effects on the adrenergic and dopaminergic systems.[4][5]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
3-(2-Aminoethyl)phenol | |
| Other names
m-Tyramine; 3-Tyramine; 3-Hydroxyphenylethylamine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.197.155 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H11NO | |
| Molar mass | 137.182 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
meta-Tyramine is produced in humans via aromatic amino acid decarboxylase-mediated metabolism of meta-tyrosine.[6] meta-Tyramine can be metabolized into dopamine via peripheral or brain CYP2D6 enzymes in humans.[7]
See also
References
- Boulton AA, Huebert ND (November 1981). "Biosynthesis of some urinary trace amines in the rat and the human". Research Communications in Chemical Pathology and Pharmacology. 34 (2): 295–310. PMID 7335956.
- Dyck LE, Juorio AV, Boulton AA (June 1982). "The in vitro release of endogenous m-tyramine, p-tyramine and dopamine from rat striatum". Neurochemical Research. 7 (6): 705–16. doi:10.1007/bf00965523. PMID 7121718.
- Sardar A, Juorio AV, Boulton AA (June 1987). "The concentration of p- and m-tyramine in the rat mesolimbic system: its regional distribution and effect of monoamine oxidase inhibition". Brain Research. 412 (2): 370–4. doi:10.1016/0006-8993(87)91145-0. PMID 3607473.
- Dyck LE, Kazakoff CW, Dourish CT (October 1982). "The role of catecholamines, 5-hydroxytryptamine and m-tyramine in the behavioural effects of m-tyrosine in the rat". European Journal of Pharmacology. 84 (3–4): 139–49. doi:10.1016/0014-2999(82)90196-0. PMID 7173317.
- McQuade PS, Wood PL (1984). "The effects of administration of meta-tyramine and para-tyramine on dopamine and its metabolites in the rat striatum". Progress in Neuro-psychopharmacology & Biological Psychiatry. 8 (4–6): 705–9. doi:10.1016/0278-5846(84)90042-3. PMID 6531442.
- "EC 4.1.1.28 – Aromatic-L-amino-acid decarboxylase (Homo sapiens)". BRENDA. Technische Universität Braunschweig. July 2016. Retrieved 7 October 2016.
Substrate: m-tyrosine
Product: m-tyramine + CO2
Organism: Homo sapiens
Reaction diagram - Wang X, Li J, Dong G, Yue J (February 2014). "The endogenous substrates of brain CYP2D". Eur. J. Pharmacol. 724: 211–218. doi:10.1016/j.ejphar.2013.12.025. PMID 24374199.
The highest level of brain CYP2D activity was found in the substantia nigra ... The in vitro and in vivo studies have shown the contribution of the alternative CYP2D-mediated dopamine synthesis to the concentration of this neurotransmitter although the classic biosynthetic route to dopamine from tyrosine is active. ... Tyramine levels are especially high in the basal ganglia and limbic system, which are thought to be related to individual behavior and emotion (Yu et al., 2003c). ... Rat CYP2D isoforms (2D2/2D4/2D18) are less efficient than human CYP2D6 for the generation of dopamine from p-tyramine. The Km values of the CYP2D isoforms are as follows: CYP2D6 (87–121 μm) ≈ CYP2D2 ≈ CYP2D18 > CYP2D4 (256 μm) for m-tyramine and CYP2D4 (433 μm) > CYP2D2 ≈ CYP2D6 > CYP2D18 (688 μm) for p-tyramine
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.