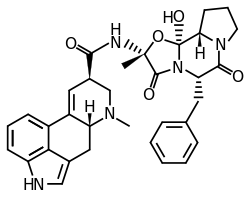
Ergotamine
Ergotamine is an ergopeptine and part of the ergot family of alkaloids; it is structurally and biochemically closely related to ergoline. It possesses structural similarity to several neurotransmitters, and has biological activity as a vasoconstrictor.
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Cafergot, Ergomar |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Intravenous: 100%,[1] Intramuscular: 47%,[2] Oral: <1%[3] (Enhanced by co-administration of caffeine[1]) |
| Metabolism | Hepatic[2] |
| Elimination half-life | 2 hours[2] |
| Excretion | 90% biliary[2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.003.658 |
| Chemical and physical data | |
| Formula | C33H35N5O5 |
| Molar mass | 581.673 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
It is used medicinally for treatment of acute migraine attacks (sometimes in combination with caffeine). Medicinal usage of ergot fungus began in the 16th century to induce childbirth, yet dosage uncertainties discouraged the use. It has been used to prevent post-partum hemorrhage (bleeding after childbirth). It was first isolated from the ergot fungus by Arthur Stoll at Sandoz in 1918 and marketed as Gynergen in 1921.[4]
Mechanism of action
The 5-hydroxytryptamine (5-HT) receptor family mediates the effects of several drugs highly effective in migraine primarily by activating 5-HT(1B) , 5-HT(1D) , and 5-HT(1F) receptors. Ergotamine, dihydroergotamine, and methysergide, as well as the "triptan" sumatriptan, are all agonists for these receptors. Ergotamine and DHE were originally developed as sympatholytics, but it was later concluded that their therapeutic efficacy was probably mediated by vasoconstriction of cranial blood vessels. As expected from this pharmacologic profile, their most important pharmacologic effect is arterial constriction.[5]
Biosynthesis
Ergotamine is a secondary metabolite (natural product) and the principal alkaloid produced by the ergot fungus, Claviceps purpurea, and related fungi in the family Clavicipitaceae.[6] Its biosynthesis in these fungi requires the amino acid L-tryptophan and dimethylallyl diphosphate. These precursor compounds are the substrates for the enzyme, tryptophan dimethylallyltransferase, catalyzing the first step in ergot alkaloid biosynthesis, i.e., the prenylation of L-tryptophan. Further reactions, involving methyltransferase and oxygenase enzymes, yield the ergoline, lysergic acid. Lysergic acid (LA) is the substrate of lysergyl peptide synthetase, a nonribosomal peptide synthetase, which covalently links LA to the amino acids, L-alanine, L-proline, and L-phenylalanine. Enzyme-catalyzed or spontaneous cyclizations, oxygenations/oxidations, and isomerizations at selected residues precede, and give rise to, formation of ergotamine.[7]
Medical use
Ergotamine continues to be prescribed for migraines and cluster headaches.[8]
Contraindications
Contraindications include: atherosclerosis, Buerger's syndrome, coronary artery disease, hepatic disease, pregnancy, pruritus, Raynaud's syndrome, and renal disease.[9] It's also contraindicated if patient is taking macrolide antibiotics (e.g., erythromycin), certain HIV protease inhibitors (e.g., ritonavir, nelfinavir, indinavir), certain azole antifungals (e.g., ketoconazole, itraconazole, voriconazole) delavirdine, efavirenz or a 5-HT1 agonist (e.g., sumatriptan). [10]
Availability and dosage
In the United States, ergotamine is available as a suppository, a sublingual tablet, and a tablet, sometimes in combination with caffeine. The suppository is available under the brand name Migergot, which contains 2 mg of ergotamine with 100 mg caffeine. The sublingual tablet is available under the brand name Ergomar and contains 2 mg of ergotamine. The combination tablet in combination with caffeine called Cafergot contains 1 mg of ergotamine and 100 mg of caffeine.[11]
This preparation may be used immediately following the aura/onset of pain to abort the migraine. For the best results, dosage should start at the first sign of an attack.[12]
Side effects
Side effects of ergotamine include nausea and vomiting. At higher doses, it can cause raised arterial blood pressure, vasoconstriction (including coronary vasospasm) and bradycardia or tachycardia. Severe vasoconstriction may cause symptoms of intermittent claudication.[13][8]
Legal status
Ergotamine is a controlled substance in the United States as it is a commonly used precursor for the production of LSD.[14]
See also
- Dihydroergotamine, a semi-synthetic form used as an abortive migraine treatment
- Ergotism
- Ergometrine
References
- Sanders SW, Haering N, Mosberg H, Jaeger H (1986). "Pharmacokinetics of ergotamine in healthy volunteers following oral and rectal dosing". European Journal of Clinical Pharmacology. 30 (3): 331–4. doi:10.1007/BF00541538. PMID 3732370.
- Tfelt-Hansen P, Johnson ES (1993). "Ergotamine". In Olesen J, Tfelt-Hansen P, Welch KM (eds.). The Headaches. New York: Raven Press. pp. 313–22.
- Ibraheem JJ, Paalzow L, Tfelt-Hansen P (December 1983). "Low bioavailability of ergotamine tartrate after oral and rectal administration in migraine sufferers". British Journal of Clinical Pharmacology. 16 (6): 695–9. doi:10.1111/j.1365-2125.1983.tb02243.x. PMC 1428366. PMID 6419759.
- AJ Giannini, AE Slaby. Drugs of Abuse. Oradell, NJ, Medical Economics Books, 1989.
- Dahlöf C, Maassen Van Den Brink A. (2012). "Dihydroergotamine, ergotamine, methysergide and sumatriptan - basic science in relation to migraine treatment". Headache. doi:10.1111/j.1526-4610.2012.02124.x. hdl:1765/73130. PMID 22444161.CS1 maint: uses authors parameter (link)
- "Pharmacognosy of Ergot (Argot or St. Anthony's Fire)". pharmaxchange.info. 30 December 2011. Archived from the original on 17 July 2012.
- Schardl CL, Panaccione DG, Tudzynski P (2006). "Ergot alkaloids--biology and molecular biology". The Alkaloids. Chemistry and Biology. 63: 45–86. doi:10.1016/S1099-4831(06)63002-2. ISBN 978-0-12-469563-4. PMID 17133714.
- Zajdel P, Bednarski M, Sapa J, Nowak G (2015). "Ergotamine and nicergoline - facts and myths". Pharmacol Rep. doi:10.1016/j.pharep.2014.10.010. PMID 25712664.CS1 maint: uses authors parameter (link)
- Giannini AJ (1986). Biological Foundations of Clinical Psychiatry. Oradell, NJ: Medical Economics Puclishing Co.
- "Ergotamine: Indications, Side Effects, Warnings". Drugs.com. Archived from the original on 25 March 2017. Retrieved 25 March 2017.
- "Approved Drug Products". FDA Orange Book (40th ed.). U.S. Food and Drug Administration. 2020.
- "CAFERGOT- ergotamine tartrate and caffeine tablet, film coated". DailyMed. U.S. National Library of Medicine. Archived from the original on 2014-01-16.
- "Medihaler Ergotamine". drugs.com. Archived from the original on 2016-04-01. Retrieved 2016-05-20.
- "Lists of: Scheduling Actions, Controlled Substances, Regulated Chemicals" (PDF). Drug Enforcement Administration, Diversion Control Division, Drug & Chemical Evaluation Section. U.S. Department of Justice. February 2020.