Thiamphenicol
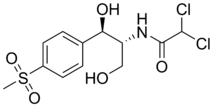
Thiamphenicol (also known as thiophenicol and dextrosulphenidol) is an antibiotic.[1] It is the methyl-sulfonyl analogue of chloramphenicol and has a similar spectrum of activity, but is 2.5 to 5 times as potent. Like chloramphenicol, it is insoluble in water, but highly soluble in lipids. It is used in many countries as a veterinary antibiotic, but is available in China, Morocco and Italy for use in humans. Its main advantage over chloramphenicol is that it has never been associated with aplastic anaemia.
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | IV, IM, oral |
| ATC code | |
| Pharmacokinetic data | |
| Metabolism | hepatic |
| Elimination half-life | 5.0 hours |
| Excretion | renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.035.762 |
| Chemical and physical data | |
| Formula | C12H15Cl2NO5S |
| Molar mass | 356.21 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 164.3 to 166.3 °C (327.7 to 331.3 °F) |
| |
| |
| | |
Thiamphenicol is also widely used in Brazil, particularly for the treatment of sexually transmitted infections and pelvic inflammatory disease.[2]
Unlike chloramphenicol, thiamphenicol is not readily metabolized in cattle, poultry, sheep, or humans, but is predominantly excreted unchanged. In pigs and rats the drug is excreted both as parent drug and as thiamphenicol glucuronate (FAO, 1997).
References
- A. Fisch, A. Bryskier (2005). "Chapter 33 : Phenicols". Antimicrobial Agents. American Society for Microbiology. doi:10.1128/9781555815929.ch33.
- Fuchs FD (2004). "Tetraciclinas e cloranfenicol". In Fuchs FD, Wannmacher L, Ferreira MB (eds.). Farmacologia clínica: fundamentos da terapêutica racional (in Portuguese) (3rd ed.). Rio de Janeiro: Guanabara Koogan. pp. 375. ISBN 0-7216-5944-6.
External links
- FAO Food and Nutrition Papers Overview at World Health Organization - Food and Agriculture Organization (1997).
- Raymond J, Boutros N, Bergeret M (2004). "Role of thiamphenicol in the treatment of community-acquired lung infections". Med Trop (Mars). 64 (1): 33–8. PMID 15224555.
- Marchese A, Debbia E, Tonoli E, Gualco L, Schito A (2002). "In vitro activity of thiamphenicol against multiresistant Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus in Italy". J Chemother. 14 (6): 554–61. PMID 12583545.