Ononin
 | |
| Names | |
|---|---|
| IUPAC name
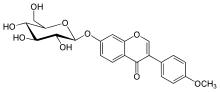
3-(4-Methoxyphenyl)-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxychromen-4-one | |
| Other names
Formononetin glucoside Formononetin-7-glucoside Formononetin 7-O-glucoside | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| |
| |
| Properties | |
| C22H22O9 | |
| Molar mass | 430.41 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ononin is an isoflavone glycoside, the 7-0-beta-D-glucopyranoside of formononetin,[1] which in turn is the 4'-O-methoxy derivative of the parent isoflavone daidzein.
Natural sources
Ononin is a major isoflavone [2] found in a number of plants and herbs like soybean [3] and Glycyrrhiza uralensis.[4]
Pharmacokinetics
Intestinal bacterial metabolic pathways may include demethylation and deglycosylation.[5] It follows that formation of formononetin and/or daidzein is possible.
Pharmacodynamics
An in vitro anti-inflammatory effect on lipopolysaccharide (LPS)-induced inflammation has been demonstrated in one study.[6]
References
- ↑ "Chinese Materia Medica: Chemistry, Pharmacology and Applications" By You-Ping Zhu, page 622, ISBN 9057022850
- ↑ PMID 28095349
- ↑ Stanley F. Osman; William F. Fett (1983). "Isoflavone glucoside stress metabolites of soybean leaves". Phytochemistry. 2 (9): 1921–1923. doi:10.1016/0031-9422(83)80013-2.
- ↑ Tsutomu Nakanishi; Akira Inada; Kazuko Kambayashia; Kaisuke Yonedaa (1985). "Flavonoid glycosides of the roots of Glycyrrhiza uralensis". Phytochemistry. 24 (2): 339–341. doi:10.1016/S0031-9422(00)83548-7.
- ↑ PMID 25322559
- ↑ PMID 28095349
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.