Hesperetin
 | |
| Names | |
|---|---|
| IUPAC name
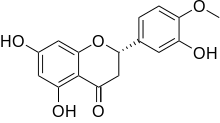
(S)-2,3-Dihydro-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4H-1-benzopyran-4-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.007.538 |
| EC Number | 208-290-2 |
| KEGG | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C16H14O6 | |
| Molar mass | 302.28 g·mol−1 |
| Melting point | 226–228 °C (439–442 °F; 499–501 K) |
| Solubility in other solvents | Sol. EtOH, alkalis |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Hesperetin is the 4'-methoxy derivative of eriodictyol, a flavanone. Hesperetin's 7-O-glycoside, hesperidin, is a naturally occurring flavanon-glycoside, the main flavonoid in lemons and sweet oranges.[1] Hesperetin (and naringenin, the parent flavanone of naringin) are not found to a significant extent in Citrus spp.[2]
Glycosides
A variety of glycosides of hesperetin are known, including:
- Hesperidin (hesperetin-7-O-rutinoside) is a water-insoluble flavonoid glycoside whose solubility is below 5 μg/ml in water.[3] Hesperidin is found in citrus fruits and upon ingestion it releases its aglycone, hesperetin.
- Neohesperidin is the 7-O-neohesperidoside of hesperetin.
- Hesperetin-7-O-α-L-Rhamnopyranoside (CAS 66513-83-5) is found in the roots of clammy cherry [4] (Cordia obliqua a.k.a. Cordia obliqua var. wallichii[5]).
Metabolism
Hesperidin 6-O-α-L-rhamnosyl-β-D-glucosidase is an enzyme that uses hesperidin and H2O to produce hesperetin and rutinose. It is found in the hyphomycetes species Stilbella fimetaria.
References
- ↑ https://pubchem.ncbi.nlm.nih.gov/compound/hesperetin
- ↑ Lewinsohn, E; Britsch, L; Mazur, Y; Gressel, J (1989). "Flavanone Glycoside Biosynthesis in Citrus: Chalcone Synthase, UDP-Glucose:Flavanone-7-O-Glucosyl-Transferase and -Rhamnosyl-Transferase Activities in Cell-Free Extracts". Plant Physiology. 91 (4): 1323–1328. doi:10.1104/pp.91.4.1323. PMC 1062186. PMID 16667183.
- ↑ Majumdar S.; Srirangam, R. (2009). "Solubility, stability, physicochemical characteristics and in vitro ocular tissue permeability of hesperidin: a natural bioflavonoid". Pharm Res. 26: 1217–1225. doi:10.1007/s11095-008-9729-6. PMC 2664388.
- ↑ http://ccd.chemnetbase.com/AAA00.entry?parentCHNumber=CNB06-R:CNB07-S
- ↑ http://www.newcropslisting.info/listing/species_pages_C/Cordia_obliqua.htm
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.