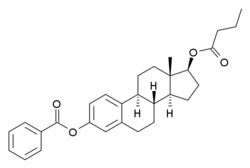
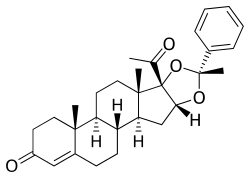
Estradiol benzoate butyrate/algestone acetophenide
 | |
 | |
| Combination of | |
|---|---|
| Estradiol benzoate butyrate | Estrogen |
| Algestone acetophenide | Progestogen |
| Clinical data | |
| Trade names | Redimen, Soluna, Unijab |
| Synonyms | Estradiol benzoate butyrate/dihydroxyprogesterone acetophenide; EBB/DHPA; Unimens |
| Routes of administration | Intramuscular injection |
Estradiol benzoate butyrate/algestone acetophenide, also known as estradiol benzoate butyrate/dihydroxyprogesterone acetophenide (EBB/DHPA) and sold under the brand names Redimen, Soluna, and Unijab, is a form of combined injectable birth control.[1][2][3][4][5][6] It contains 10 mg estradiol benzoate butyrate (EBB), an estrogen, and 150 mg algestone acetophenide (dihydroxyprogesterone acetophenide; DHPA), a progestin.[2][3] The medication is given once per month by injection into muscle.[3] It is marketed in Peru and Singapore.[2][3]
EBB/DHPA was originally developed under the tentative brand name Unimens, but ultimately was not marketed under this particular brand name.[1][7][8][9][10]
EBB has a shorter duration than estradiol enantate of about 3 weeks and hence EBB/DHPA was developed because it was thought that it would be more suitable for use as a once-monthly combined injectable contraceptive than estradiol enantate/algestone acetophenide.[11]
EBB/DHPA has relatively weak estrogenic activity and is described as progestogen-dominant.[1]
See also
References
- 1 2 3 Toppozada M (June 1977). "The clinical use of monthly injectable contraceptive preparations". Obstet Gynecol Surv. 32 (6): 335–47. doi:10.1097/00006254-197706000-00001. PMID 865726.
- 1 2 3 IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer (2007). Combined Estrogen-progestogen Contraceptives and Combined Estrogen-progestogen Menopausal Therapy. World Health Organization. pp. 433, 467. ISBN 978-92-832-1291-1.
- 1 2 3 4 IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer (1 January 1999). Hormonal Contraception and Post-menopausal Hormonal Therapy (PDF). IARC. p. 65. ISBN 978-92-832-1272-0.
- ↑ https://www.datosperu.org/farmaco-soluna-rs-N12759.php
- ↑ http://www.mims.com/singapore/drug/info/unijab
- ↑ http://www.corporacionmisalud.com/sistema/vademecum/PLM/productos/32499.htm
- ↑ Mokhtar K. Toppozada (1983), Monthly Injectable Contraceptives (PDF)
- ↑ Elsayed Saad Eldin Hafez (1980). Human reproduction: conception and contraception. Harper and Row. ISBN 978-0-06-141066-6.
- ↑ Newton JR, D'arcangues C, Hall PE (1994). "A review of "once-a-month" combined injectable contraceptives". J Obstet Gynaecol (Lahore). 4 Suppl 1: S1–34. doi:10.3109/01443619409027641. PMID 12290848.
- ↑ Toppozada MK (April 1994). "Existing once-a-month combined injectable contraceptives". Contraception. 49 (4): 293–301. doi:10.1016/0010-7824(94)90029-9. PMID 8013216.
- ↑ Goldsmith, A., & Toppozada, M. (1983). Long-acting contraception. pp. 94-95 https://www.popline.org/node/423289