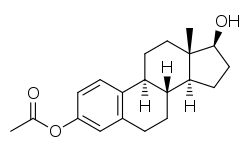
Estradiol acetate
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation |
/ˌɛstrəˈdaɪoʊl ES-trə-DY-ohl ASS-ə-tayt[1] |
| Trade names | Femtrace, Femring, Menoring |
| Synonyms | E2A; E3A; Estradiol 3-acetate |
| Routes of administration | By mouth, vaginal (ring)[2] |
| Drug class | Estrogen; Estrogen ester |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard |
100.110.039 |
| Chemical and physical data | |
| Formula | C20H26O3 |
| Molar mass | 314.419 g/mol |
| 3D model (JSmol) | |
| |
| |
Estradiol acetate, sold under the brand names Femtrace, Femring, and Menoring, is an estrogen medication which is used in hormone therapy for the treatment of menopausal symptoms in women.[3][4][5][6] It is taken by mouth or given as a vaginal ring once every three months.[2]
Side effects of estradiol acetate include breast tenderness, breast enlargement, nausea, headache, and fluid retention.[7][5][6] Estradiol acetate is a synthetic estrogen and hence is an agonist of the estrogen receptor (ER), the biological target of estrogens like estradiol.[8][9] It is an estrogen ester and a prodrug of estradiol in the body.[9][8] Because of this, it is considered to be a natural and bioidentical form of estrogen.[9]
Estradiol acetate was introduced for medical use in 2001.[10] It is available in the United States and the United Kingdom.[10][3] The formulation for use by mouth has been discontinued in the United States.[11]
Medical uses
Estradiol acetate is used as a component of menopausal hormone therapy to treat and prevent menopausal symptoms such as hot flashes and osteoporosis in women.[12][13][14][15]
The Women's Health Initiative studies report increased health risks for menopausal women when using unopposed estrogens.[6] Estrogens with or without progestins should be prescribed at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman.[6]
Available forms
Estradiol acetate comes in the form of 0.45, 0.9, and 1.8 mg oral tablets (Femtrace) and in the form of 12.4 or 24.8 mg vaginal rings that release 50 or 100 μg/day estradiol for 3 months (Femring, Menoring).[5][6][16] However, the Femtrace product was discontinued in the United States.[11]
Side effects
The side effects of estradiol acetate are the same as those of estradiol. Examples of such side effects include breast tenderness and enlargement, nausea, bloating, edema, headache, and melasma.[7]
Pharmacology
Pharmacodynamics
Estradiol acetate is an estradiol ester, or a prodrug of estradiol.[9][8] As such, it is an estrogen, or an agonist of the estrogen receptors.[8][9] Estradiol acetate is of about 15% higher molecular weight than estradiol due to the presence of its C3 acetate ester.[3] Because estradiol acetate is a prodrug of estradiol, it is considered to be a natural and bioidentical form of estrogen.[9]
Pharmacokinetics
Estradiol acetate is converted into estradiol in the body.[9][8]
Chemistry
Estradiol acetate is a synthetic estrane steroid and the C3 acetate ester of estradiol.[3] It is also known as estradiol 3-acetate or as estra-1,3,5(10)-triene-3,17β-diol 3-acetate.[3] Another common ester of estradiol in use for oral administration is estradiol valerate, which is a C17β ester of estradiol.[8][17]
History
Estradiol acetate is relatively recent to the market, having been first approved in a vaginal ring formulation as Menoring in the United Kingdom in 2001,[12] followed by a vaginal ring formulation as Femring in the United States in 2002,[2] and finally as an oral preparation as Femtrace in the United States in 2004.[2][10]
Society and culture
Generic names
Estradiol acetate is the generic name of the drug and its USAN.[3]
Brand names
Estradiol acetate is marketed under the brand names Femtrace, Femring, and Menoring.[3][18][19]
Availability
Estradiol acetate is available in the United States and the United Kingdom.[10][3]
References
- ↑ https://www.drugs.com/cdi/estradiol-acetate.html
- 1 2 3 4 Sivanandy MS, Masimasi N, Thacker HL (May 2007). "Newer hormonal therapies: lower doses; oral, transdermal, and vaginal formulations". Cleveland Clinic Journal of Medicine. 74 (5): 369–75. doi:10.3949/ccjm.74.5.369. PMID 17506242.
- 1 2 3 4 5 6 7 8 https://www.drugs.com/international/estradiol-acetate.html
- ↑ Buckler H, Al-Azzawi F (2003). "The effect of a novel vaginal ring delivering oestradiol acetate on climacteric symptoms in postmenopausal women". BJOG. 110 (8): 753–9. doi:10.1016/s1470-0328(03)02908-2. PMID 12892687.
- 1 2 3 https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021633s005lbl.pdf
- 1 2 3 4 5 "FEMRING". DailyMed. U.S. National Library of Medicine.
- 1 2 Amit K. Ghosh (23 September 2010). Mayo Clinic Internal Medicine Board Review. OUP USA. pp. 222–. ISBN 978-0-19-975569-1.
- 1 2 3 4 5 6 Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- 1 2 3 4 5 6 7 Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. p. 261. ISBN 978-3-642-60107-1.
Natural estrogens considered here include: [...] Esters of 17β-estradiol, such as estradiol valerate, estradiol benzoate and estradiol cypionate. Esterification aims at either better absorption after oral administration or a sustained release from the depot after intramuscular administration. During absorption, the esters are cleaved by endogenous esterases and the pharmacologically active 17β-estradiol is released; therefore, the esters are considered as natural estrogens.
- 1 2 3 4 Ballagh SA (2004). "Vaginal rings for menopausal symptom relief". Drugs Aging. 21 (12): 757–66. doi:10.2165/00002512-200421120-00001. PMID 15382956.
- 1 2 https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=021633
- 1 2 Speroff L (October 2003). "Efficacy and tolerability of a novel estradiol vaginal ring for relief of menopausal symptoms". Obstetrics and Gynecology. 102 (4): 823–34. doi:10.1016/s0029-7844(03)00764-6. PMID 14551014.
- ↑ Al-Azzawi F, Lees B, Thompson J, Stevenson JC (2005). "Bone mineral density in postmenopausal women treated with a vaginal ring delivering systemic doses of estradiol acetate". Menopause (New York, N.Y.). 12 (3): 331–9. doi:10.1097/01.gme.0000163870.03388.4d. PMID 15879923.
- ↑ Utian WH, Speroff L, Ellman H, Dart C (2005). "Comparative controlled trial of a novel oral estrogen therapy, estradiol acetate, for relief of menopause symptoms". Menopause (New York, N.Y.). 12 (6): 708–15. doi:10.1097/01.gme.0000184220.63459.a8. PMID 16278614.
- ↑ Speroff L, Haney AF, Gilbert RD, Ellman H (2006). "Efficacy of a new, oral estradiol acetate formulation for relief of menopause symptoms". Menopause (New York, N.Y.). 13 (3): 442–50. doi:10.1097/01.gme.0000182802.06762.b2. PMID 16735941.
- ↑ Deitra Leonard Lowdermilk; Shannon E. Perry; Mary Catherine Cashion; Kathryn Rhodes Alden (18 December 2014). Maternity and Women's Health Care - E-Book. Elsevier Health Sciences. pp. 137–. ISBN 978-0-323-39019-4.
- ↑ Düsterberg B, Nishino Y (December 1982). "Pharmacokinetic and pharmacological features of oestradiol valerate". Maturitas. 4 (4): 315–24. doi:10.1016/0378-5122(82)90064-0. PMID 7169965.
- ↑ U.S. Food and Drug Administration (2009). Menopause - Medicines to Help You. GPO FCIC. pp. 3–. ISBN 978-1-61221-026-1.
- ↑ Marc A. Fritz; Leon Speroff (28 March 2012). Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. pp. 757–. ISBN 978-1-4511-4847-3.