''n''-Butanol
| |||
 | |||
| Names | |||
|---|---|---|---|
| Systematic IUPAC name
Butan-1-ol[1] | |||
| Other names
Butalcohol Butanol | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 3DMet | B00907 | ||
| 969148 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.683 | ||
| EC Number | 200-751-6 | ||
| 25753 | |||
| KEGG | |||
| MeSH | 1-Butanol | ||
PubChem CID |
|||
| RTECS number | EO1400000 | ||
| UNII | |||
| UN number | 1120 | ||
| |||
| |||
| Properties | |||
| C4H10O | |||
| Molar mass | 74.12 g·mol−1 | ||
| Appearance | Colourless, refractive liquid | ||
| Odor | banana-like,[2] harsh, alcoholic and sweet | ||
| Density | 0.81 g cm−3 | ||
| Melting point | −89.8 °C (−129.6 °F; 183.3 K) | ||
| Boiling point | 117.7 °C (243.9 °F; 390.8 K) | ||
| 73 g L−1 at 25 °C | |||
| Solubility | very soluble in acetone miscible with ethanol, ethyl ether | ||
| log P | 0.839 | ||
| Vapor pressure | 6 mmHg (20 °C)[3] | ||
| Acidity (pKa) | 16.10 | ||
| -56.536·10−6 cm3/mol | |||
Refractive index (nD) |
1.3993 (20 °C) | ||
| Viscosity | 2.573 mPa×s (at 25 °C) [4] | ||
| 1.66 D | |||
| Thermochemistry | |||
Std molar entropy (S |
225.7 J K−1 mol−1 | ||
Std enthalpy of formation (ΔfH |
−328(4) kJ mol−1 | ||
Std enthalpy of combustion (ΔcH |
−2670(20) kJ mol−1 | ||
| Hazards | |||
| Safety data sheet | ICSC 0111 | ||
| GHS pictograms |  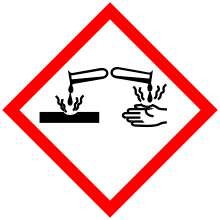  | ||
| NFPA 704 | |||
| Flash point | 35 °C (95 °F; 308 K) | ||
| 343 °C (649 °F; 616 K) | |||
| Explosive limits | 1.45–11.25% | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
790 mg/kg (rat, oral) | ||
LDLo (lowest published) |
3484 mg/kg (rabbit, oral) 790 mg/kg (rat, oral) 1700 mg/kg (dog, oral)[5] | ||
LC50 (median concentration) |
9221 ppm (mammal) 8000 ppm (rat, 4 hr)[5] | ||
| US health exposure limits (NIOSH): | |||
PEL (Permissible) |
TWA 100 ppm (300 mg/m3)[3] | ||
REL (Recommended) |
C 50 ppm (150 mg/m3) [skin][3] | ||
IDLH (Immediate danger) |
1400 ppm[3] | ||
| Related compounds | |||
Related compounds |
Butanethiol n-Butylamine Diethyl ether Pentane | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
n-Butanol or n-butyl alcohol or normal butanol is a primary alcohol with a 4-carbon structure and the chemical formula C4H9OH. Its isomers include isobutanol, 2-butanol, and tert-butanol. Butanol is one of the group of "fusel alcohols" (from the German for "bad liquor"), which have more than two carbon atoms and have significant solubility in water.[6]
n-Butanol occurs naturally as a minor product of the fermentation of sugars and other carbohydrates,[7] and is present in many foods and beverages.[8][9] It is also a permitted artificial flavorant in the United States,[10] used in butter, cream, fruit, rum, whiskey, ice cream and ices, candy, baked goods and cordials.[11] It is also used in a wide range of consumer products.[8]
The largest use of n-butanol is as an industrial intermediate, particularly for the manufacture of butyl acetate (itself an artificial flavorant and industrial solvent). It is a petrochemical, manufactured from propylene and usually used close to the point of manufacture. Estimated production figures for 1997 are: United States 784,000 tonnes; Western Europe 575,000 tonnes; Japan 225,000 tonnes.[9]
Isomers
The unmodified term butanol usually refers to the straight chain isomer with the alcohol functional group at the terminal carbon, which is also known as n-butanol or 1-butanol. The straight chain isomer with the alcohol at an internal carbon is sec-butanol or 2-butanol. The branched isomer with the alcohol at a terminal carbon is isobutanol or 2-methyl-1-propanol, and the branched isomer with the alcohol at the internal carbon is tert-butanol or 2-methyl-2-propanol.
 |
 |
 | |
| n-butanol | sec-butanol | isobutanol | tert-butanol |
The butanol isomers have different melting and boiling points. n-butanol and isobutanol have limited solubility, sec-butanol has substantially greater solubility, while tert-butanol is fully miscible with water above tert-butanol's melting point. The hydroxyl group makes the molecule polar, promoting solubility in water, while the longer hydrocarbon chain mitigates the polarity and reduces solubility. The shorter chain molecules of methanol, ethanol, propanol, and tert-butanol are fully miscible with water, while n-butanol is only moderately soluble because of the diminishing polarity in the longer hydrocarbon group.
Production
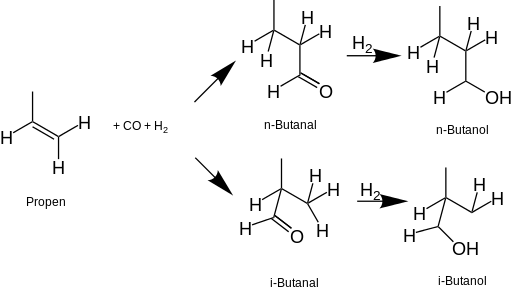
Since the 1950s, most n-Butanol in the United States is produced industrially from fossil fuels. The most common process starts with propene, which is put through a hydroformylation reaction (oxo process) to form butanal (butyraldehyde) in the presence of a rhodium-based homogeneous catalyst similar to Wilkinson's catalyst. The butyraldehyde is then hydrogenated to produce n-butanol.[9]. tert-butanol is derived from isobutane as a co-product of propylene oxide production.
Butanol can also be produced by fermentation of biomass by bacteria. Prior to the 1950s, Clostridium acetobutylicum was used in industrial fermentation to produce butanol. Research in the past few decades showed results of other microorganisms that can produce butanol through fermentation.
Industrial use
n-butanol is an intermediate in the production of butyl acrylate, butyl acetate, dibutyl phthalate, dibutyl sebacate, and other butyl esters,[12][13] butyl ethers such as ethylene glycol monobutyl ether, di- and triethylene glycol monobutyl ether, and the corresponding butyl ether acetates. Other industrial uses include the manufacture of pharmaceuticals, polymers, pyroxylin plastics, herbicide esters, printing (e.g., 2,4-D, 2,4,5-T)[14] and butyl xanthate. It is also used as a diluent/reactant in the manufacture of urea–formaldehyde and melamine–formaldehyde resins.[9]
Butanol is used as a solvent for a wide variety of chemical and textile processes, in organic synthesis, and as a chemical intermediate. It is also used as a paint thinner and a solvent in other coating applications where a relatively slow evaporating latent solvent is preferable, as with lacquers and ambient-cured enamels. It is also used as a component of hydraulic and brake fluids.[15]
Butanol is used in the synthesis of 2-butoxyethanol. A major application for butanol is as a reactant with acrylic acid to produce butyl acrylate, a primary ingredient of water based acrylic paint.[16]
It is also used as a base for perfumes, but on its own has a highly alcoholic aroma.
Salts of butanol are chemical intermediates; for example, alkali metal salts of tert-butanol are tert-butoxides.
Other uses
n-Butanol is used as an ingredient in perfumes and as a solvent for the extraction of essential oils.[12] n-Butanol is also used as an extractant in the manufacture of antibiotics, hormones, and vitamins;[12][13] a solvent for paints, coatings, natural resins, gums, synthetic resins, dyes, alkaloids, and camphor.[12][13] Other miscellaneous applications of n-butanol are as a swelling agent in textiles, as a component of hydraulic brake fluids, cleaning formulations, degreasers, and repellents;[8] and as a component of ore floation agents,[14] and of wood-treating systems.[17]
n-Butanol has been proposed as a substitute for diesel fuel and gasoline. It is produced in small quantities in nearly all fermentations (see fusel oil), but species of Clostridium produce much higher yields of butanol, and research is currently underway to increase the ultimate yield of biobutanol from biomass.
Butanol is considered as a potential biofuel (butanol fuel). Butanol at 85 percent strength can be used in cars designed for gasoline (petrol) without any change to the engine (unlike 85% ethanol), and it contains more energy for a given volume than ethanol and almost as much as gasoline, and a vehicle using butanol would return fuel consumption more comparable to gasoline than ethanol. Butanol can also be added to diesel fuel to reduce soot emissions.[18]
The production or, in some cases, use of the following substances may result in exposure to n-butanol: artificial leather, butyl esters, rubber cement, dyes, fruit essences, lacquers, motion picture, and photographic films, raincoats, perfumes, pyroxylin plastics, rayon, safety glass, shellac varnish, and waterproofed cloth.[8]
Occurrence in nature
Honey bees use n-butanol as an Alarm pheromone.
Occurrence in food
n-Butanol occurs naturally as a result of carbohydrate fermentation in a number of alcoholic beverages, including beer,[19] grape brandies,[20] wine,[21] and whisky.[22] It has been detected in the volatiles of hops,[23] jack fruit,[24] heat-treated milks,[25] musk melon,[26] cheese,[27] southern pea seed,[28] and cooked rice.[29] n-Butanol is also formed during deep frying of corn oil, cottonseed oil, trilinolein, and triolein.[30]
n-Butanol is used as an ingredient in processed and artificial flavourings,[12] and for the extraction of lipid-free protein from egg yolk,[31] natural flavouring materials and vegetable oils, the manufacture of hop extract for beermaking, and as a solvent in removing pigments from moist curd leaf protein concentrate.[32]
Metabolism and toxicity
n-Butanol is readily absorbed through the intestinal tract and lungs, and also to some extent through the skin.[33] It is metabolized completely in vertebrates in a manner similar to ethanol: alcohol dehydrogenase converts n-butanol to butyraldehyde; this is then converted to butyric acid by aldehyde dehydrogenase. Butyric acid can be fully metabolized to carbon dioxide and water by the β-oxidation pathway. In the rat, only 0.03% of an oral dose of 2,000 mg/kg was excreted in the urine.[34]
The acute toxicity of n-butanol is relatively low, with oral LD50 values of 790–4,360 mg/kg (rat; comparable values for ethanol are 7,000–15,000 mg/kg).[9][35] No deaths were reported at an inhaled concentration of 8,000 ppm (4-hour exposure, rats). At sub-lethal doses, n-butanol acts as a depressant of the central nervous system, similar to ethanol: one study in rats indicated that the intoxicating potency of n-butanol is some six times higher than that of ethanol, possibly because of its slower transformation by alcohol dehydrogenase.[36]
n-Butanol is a natural component of many alcoholic beverages, albeit in low (but variable) concentrations.[37][38] It (along with similar fusel alcohols) is reputed to be responsible for severe hangovers, although experiments in animal models show no evidence for this.[39] An unknown dose n-Butanol was consumed by a 47-year-old male with no previous medical history, leading to a range of adverse health effects.[40]
Like many alcohols, butanol is considered toxic. It has shown low order of toxicity in single dose experiments to laboratory animals.[41][42] and is considered safe enough for use in cosmetics. Brief, repeated overexposure with the skin can result in depression of the central nervous system, as with other short-chain alcohols. Exposure may also cause severe eye irritation and moderate skin irritation. The main dangers are from prolonged exposure to fumes. In extreme cases this includes suppression of the central nervous system and even death. Under most circumstances, butanol is quickly metabolized to carbon dioxide. It has not been shown to damage DNA or cause cancer.
Other hazards
Liquid n-butanol, as is common with most organic solvents, is extremely irritating to the eyes; repeated contact with the skin can also cause irritation.[9] This is believed to be a generic effect of "defatting". No skin sensitization has been observed. Irritation of the respiratory pathways occurs only at very high concentrations (>2,400 ppm).[43]
With a flash point of 35 °C, n-butanol presents a moderate fire hazard: it is slightly more flammable than kerosene or diesel fuel but less flammable than many other common organic solvents. The depressant effect on the central nervous system (similar to ethanol intoxication) is a potential hazard when working with n-butanol in enclosed spaces, although the odour threshold (0.2–30 ppm) is far below the concentration which would have any neurological effect.[43][44]
n-Butanol is of low toxicity to aquatic vertebrates and invertebrates. It is rapidly biodegraded in water, although an estimated 83% partitions to air where it is degraded by hydroxyl radicals with a half-life of 1.2–2.3 days. It has low potential to bioaccumulate.[9] A potential hazard of significant discharges to watercourses is the rise in chemical oxygen demand (C.O.D.) associated with its biodegradation.
See also
External links
- International Chemical Safety Card 0111
- "NIOSH Pocket Guide to Chemical Hazards #0076". National Institute for Occupational Safety and Health (NIOSH).
- SIDS Initial Assessment Report for n-Butanol from the Organisation for Economic Co-operation and Development (OECD)
- IPCS Environmental Health Criteria 65: Butanols: four isomers
- IPCS Health and Safety Guide 3: 1-Butanol
References
- ↑ "1-Butanol - Compound Summary". The PubChem Project. USA: National Center of Biotechnology Information.
- ↑ [n-Butanol Product Information, The Dow Chemical Company, Form No. 327-00014-1001, page 1]
- 1 2 3 4 "NIOSH Pocket Guide to Chemical Hazards #0076". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Dubey, Gyan (2008). "Study of densities, viscosities, and speeds of sound of binary liquid mixtures of butan-1-ol with n-alkanes (C6, C8, and C10) at T = (298.15, 303.15, and 308.15) K". The Journal of Chemical Thermodynamics. 40 (2): 309–320. doi:10.1016/j.jct.2007.05.016.
- 1 2 "N-butyl alcohol". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ↑ Atsumi, S.; Hanai, T.; Liao, J. C. (2008). "Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels". Nature. 451 (7174): 86–9. doi:10.1038/nature06450. PMID 18172501.
- ↑ Hazelwood, Lucie A.; Daran, Jean-Marc; van Maris, Antonius J. A.; Pronk, Jack T.; Dickinson, J. Richard (2008), "The Ehrlich pathway for fusel alcohol production: a century of research on Saccharomyces cerevisiae metabolism", Appl. Environ. Microbiol., 74 (8): 2259–66, doi:10.1128/AEM.02625-07, PMC 2293160, PMID 18281432 .
- 1 2 3 4 Butanols: four isomers, Environmental Health Criteria monograph No. 65, Geneva: World Health Organization, 1987, ISBN 92-4-154265-9 .
- 1 2 3 4 5 6 7 n-Butanol (PDF), SIDS Initial Assessment Report, Geneva: United Nations Environment Programme, April 2005 .
- ↑ 21 C.F.R. § 172.515; 42 FR 14491, Mar. 15, 1977, as amended.
- ↑ Hall, R. L.; Oser, B. L. (1965), "Recent progress in the consideration of flavouring ingredients under the food additives amendment. III. Gras substances", Food Technol.: 151 , cited in Butanols: four isomers, Environmental Health Criteria monograph No. 65, Geneva: World Health Organization, 1987, ISBN 92-4-154265-9 .
- 1 2 3 4 5 Mellan, I. (1950), Industrial Solvents, New York: Van Nostrand Reinhold, pp. 482–88 , cited in Butanols: four isomers, Environmental Health Criteria monograph No. 65, Geneva: World Health Organization, 1987, ISBN 92-4-154265-9 .
- 1 2 3 Doolittle, A. K. (1954), The Technology of Solvents and Plasticizers, New York: Wiley, pp. 644–45 , cited in Butanols: four isomers, Environmental Health Criteria monograph No. 65, Geneva: World Health Organization, 1987, ISBN 92-4-154265-9 .
- 1 2 Monich, J. A. (1968), Alcohols: Their Chemistry, Properties, and Manufacture, New York: Chapman and Reinhold , cited in Butanols: four isomers, Environmental Health Criteria monograph No. 65, Geneva: World Health Organization, 1987, ISBN 92-4-154265-9 .
- ↑ Isobutanol at chemicalland21.com
- ↑ Harris O.; et al. (August 1998). Toxicological Profile for 2-Butoxyethanol and 2-butoxyethanol acetate. U.S. Dept of Health and Human Services.
- ↑ ZA 7801031, Amundsen, J.; R. J. Goodwin & W. H. Wetzel, "Water-soluble pentachlorophenol and tetrachlorophenol wood-treating systems", published 28 Feb. 1979.
- ↑ Antoni, D; Zverlov, V. & Schwarz, W H. (2007). "Biofuels from Microbes". Applied Microbiology and Biotechnology. 77: 23–35. doi:10.1007/s00253-007-1163-x.
- ↑ Bonte, W. (1979), "Congener substances in German and foreign beers", Blutalkohol, 16: 108–24 , cited in Butanols: four isomers, Environmental Health Criteria monograph No. 65, Geneva: World Health Organization, 1987, ISBN 92-4-154265-9 .
- ↑ Schreier, Peter; Drawert, Friedrich; Winkler, Friedrich (1979), "Composition of neutral volatile constituents in grape brandies", J. Agric. Food Chem., 27 (2): 365–72, doi:10.1021/jf60222a031 .
- ↑ Bonte, W. (1978), "Congener content of wine and similar beverages", Blutalkohol, 15: 392–404 , cited in Butanols: four isomers, Environmental Health Criteria monograph No. 65, Geneva: World Health Organization, 1987, ISBN 92-4-154265-9 .
- ↑ Postel, W.; Adam, L. (1978), "Gas chromatographic characterization of whiskey. III. Irish whiskey", Branntweinwirtschaft, 118: 404–7 , cited in Butanols: four isomers, Environmental Health Criteria monograph No. 65, Geneva: World Health Organization, 1987, ISBN 92-4-154265-9 .
- ↑ Tressl, Roland; Friese, Lothar; Fendesack, Friedrich; Koeppler, Hans (1978), "Studies of the volatile composition of hops during storage", J. Agric. Food Chem., 26 (6): 1426–30, doi:10.1021/jf60220a036 .
- ↑ Swords, G.; Bobbio, P. A.; Hunter, G. L. K. (1978), "Volatile constituents of jack fruit (Arthocarpus heterophyllus)", J. Food Sci., 43 (2): 639–40, doi:10.1111/j.1365-2621.1978.tb02375.x .
- ↑ Jaddou, Haytham A.; Pavey, John A.; Manning, Donald J. (1978), "Chemical analysis of flavor volatiles in heat-treated milks", J. Dairy Res., 45 (3): 391–403, doi:10.1017/S0022029900016617 .
- ↑ Yabumoto, K.; Yamaguchi, M.; Jennings, W. G. (1978), "Production of volatile compounds by Muskmelon, Cucumis melo", Food Chem., 3 (1): 7–16, doi:10.1016/0308-8146(78)90042-0 .
- ↑ Dumont, Jean Pierre; Adda, Jacques (1978), "Occurrence of sesquiterpones in mountain cheese volatiles", J. Agric. Food Chem., 26 (2): 364–67, doi:10.1021/jf60216a037 .
- ↑ Fisher, Gordon S.; Legendre, Michael G.; Lovgren, Norman V.; Schuller, Walter H.; Wells, John A. (1979), "Volatile constituents of southernpea seed [Vigna unguiculata (L.) Walp.]", J. Agric. Food Chem., 27 (1): 7–11, doi:10.1021/jf60221a040 .
- ↑ Yajima, Izumi; Yanai, Tetsuya; Nakamura, Mikio; Sakakibara, Hidemasa; Habu, Tsutomu (1978), "Volatile flavor components of cooked rice", Agric. Biol. Chem., 42 (6): 1229–33, doi:10.1271/bbb1961.42.1229 .
- ↑ Chang, S. S.; Peterson, K. J.; Ho, C. (1978), "Chemical reactions involved in the deep-fat frying of foods", J. Am. Oil Chem. Soc.: 718–27 , cited in Butanols: four isomers, Environmental Health Criteria monograph No. 65, Geneva: World Health Organization, 1987, ISBN 92-4-154265-9 .
- ↑ Meslar, Harry W.; White, Harold B., III (1978), "Preparation of lipid-free protein extracts of egg yolk", Anal. Biochem., 91 (1): 75–81, doi:10.1016/0003-2697(78)90817-5, PMID 9762085 .
- ↑ Bray, Walter J.; Humphries, Catherine (1978), "Solvent fractionation of leaf juice to prepare green and white protein products", J. Sci. Food Agric., 29 (10): 839–46, doi:10.1002/jsfa.2740291003 .
- ↑ Theorell, Hugo; Bonnichsen, Roger; Holtermann, Hugo; Sörensen, JöRgine Stene; Sörensen, Nils Andreas (1951), "Studies on Liver Alcohol Dehydrogenase I. Equilibria and Initial Reaction Velocities" (PDF), Acta Chem. Scand., 5: 1105–26, doi:10.3891/acta.chem.scand.05-1105 . Winer, Alfred D.; Nurmikko, V.; Hartiala, K.; Hartiala, K.; Veige, S.; Diczfalusy, E. (1958), "A Note of the Substrate Specificity of Horse Liver Alcohol Dehydrogenase" (PDF), Acta Chem. Scand., 12: 1695–96, doi:10.3891/acta.chem.scand.12-1695 . Merritt, A. Donald; Tomkins, Gordon M. (1959), "Reversible Oxidation of Cyclic Secondary Alcohols by Liver Alcohol Dehydrogenase", J. Biol. Chem., 234 (10): 2778–82 . von Wartburg, Jean-Pierre; Bethane, J. L.; Vallee, B. L. (1964), "Human Liver Alcohol Dehydrogenase: Kinetic and Physiochemical Properties", Biochemistry, 3 (11): 1775–82, doi:10.1021/bi00899a033 .
- ↑ Gaillard, D.; Derache, R. (1965), "Métabilisation de différents alcools présents dans les biossons alcooliques chez le rat", Trav. Soc. Pharmacol. Montpellier, 25: 541–62 , cited in Butanols: four isomers, Environmental Health Criteria monograph No. 65, Geneva: World Health Organization, 1987, ISBN 92-4-154265-9 .
- ↑ Ethanol (PDF), SIDS Initial Assessment Report, Geneva: United Nations Environment Programme, August 2005 .
- ↑ McCreery, N. J.; Hunt, W. A. (1978), "Physico-chemical correlates of alcohol intoxication", Neuropharmacology, 17 (7): 451–61, doi:10.1016/0028-3908(78)90050-3, PMID 567755 .
- ↑ Woo, Kang-Lyung (2005), "Determination of low molecular weight alcohols including fusel oil in various samples by diethyl ether extraction and capillary gas chromatography", J. AOAC Int., 88 (5): 1419–27, doi:10.5555/jaoi.2005.88.5.1419, PMID 16385992 .
- ↑ Lachenmeier, Dirk W.; Haupt, Simone; Schulz, Katja (2008), "Defining maximum levels of higher alcohols in alcoholic beverages and surrogate alcohol products", Regul. Toxicol. Pharmacol., 50 (3): 313–21, doi:10.1016/j.yrtph.2007.12.008, PMID 18295386 .
- ↑ Hori, Hisako; Fujii, Wataru; Hatanaka, Yutaka; Suwa, Yoshihide (2003), "Effects of fusel oil on animal hangover models", Alcohol. Clin. Exp. Res., 27 (8 Suppl): 37S–41S, doi:10.1097/01.ALC.0000078828.49740.48, PMID 12960505 .
- ↑ Bunc, M.; Pezdir, T.; Možina, H.; Možina, M.; Brvar, M. (2006), "Butanol ingestion in an airport hangar", Hum. Exp. Toxicol., 25 (4): 195–97, doi:10.1191/0960327106ht607oa, PMID 16696295 .
- ↑ 16 ECETOC JACC No. 41 n-Butanol (CAS No. 71-36-3), European Centre for Ecotoxicology and Toxicology of Chemicals, Brussels, December 2003, pages 3-4.
- ↑ n-Butanol
- 1 2 Wysocki, C. J.; Dalton, P. (1996), Odor and Irritation Thresholds for 1-Butanol in Humans, Philadelphia: Monell Chemical Senses Center , cited in n-Butanol (PDF), SIDS Initial Assessment Report, Geneva: United Nations Environment Programme, April 2005 .
- ↑ Cometto-Muñiz, J. Enrique; Cain, William S. (1998), "Trigeminal and Olfactory Sensitivity: Comparison of Modalities and Methods of Measurement", Int. Arch. Occup. Environ. Health, 71 (2): 105–10, doi:10.1007/s004200050256, PMID 9580447 .