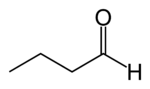
Butyraldehyde
 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Butanal | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.004.225 |
| KEGG | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C4H8O | |
| Molar mass | 72.11 g/mol |
| Appearance | colorless liquid |
| Odor | pungent, aldehyde odor |
| Density | 0.8016 g/mL |
| Melting point | −96.86 °C (−142.35 °F; 176.29 K) |
| Boiling point | 74.8 °C (166.6 °F; 347.9 K) |
| 7.6 g/100 mL (20 °C) | |
| Solubility | miscible with ethanol, ether, toluene very soluble in acetone, benzene slightly soluble in chloroform |
| log P | 0.88 |
| -46,08·10−6 cm3/mol | |
Refractive index (nD) |
1.3766 |
| Viscosity | 0.45 cP (20 °C) |
| 2.72 D | |
| Thermochemistry | |
Std enthalpy of combustion (ΔcH |
2470.34 kJ/mol |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
EU classification (DSD) (outdated) |
Flammable (F) |
| R-phrases (outdated) | R11 |
| S-phrases (outdated) | (S2), S9, S29, S33 |
| NFPA 704 | |
| Flash point | −7 °C (19 °F; 266 K) |
| 230 °C (446 °F; 503 K) | |
| Explosive limits | 1.9–12.5% |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
2490 mg/kg (rat, oral) |
| Related compounds | |
Related aldehyde |
Propionaldehyde Pentanal |
Related compounds |
Butan-1-ol Butyric acid, isobutyraldehyde |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Butyraldehyde, also known as butanal, is an organic compound with the formula CH3(CH2)2CHO. This compound is the aldehyde derivative of butane. It is a colourless flammable liquid with an acrid smell. It is miscible with most organic solvents.
Production
Butyraldehyde is produced almost exclusively by the hydroformylation of propylene:
- CH3CH=CH2 + H2 + CO → CH3CH2CH2CHO
Traditionally, hydroformylation was catalyzed by cobalt carbonyl and later rhodium complexes of triphenylphosphine. The dominant technology involves the use of rhodium catalysts derived from the water-soluble ligand Tppts. An aqueous solution of the rhodium catalyst converts the propylene to the aldehyde, which forms a lighter immiscible phase. About 6 billion kilograms are produced annually by hydroformylation. A significant application is its conversion to 2-ethylhexanol for production of plasticizers.
_phthalate.svg.png)
Butyraldehyde can be produced by the catalytic dehydrogenation of n-butanol. At one time, it was produced industrially by the catalytic hydrogenation of crotonaldehyde, which is derived from acetaldehyde.[2]
Upon prolonged exposure to air, butyraldehyde oxidizes to form butyric acid.
References
- ↑ Merck Index, 11th Edition, 1591
- ↑ Boy Cornils, Richard W. Fischer, Christian Kohlpaintner "Butanals" in Ullmann's Encyclopedia of Industrial Chemistry, 2000, Wiley-VCH, Weinheim. doi:10.1002/14356007.a04_447
