BNC-210
BNC210 (also known as IW-2143 during its time licensed to Ironwood Pharmaceuticals) is an anxiolytic drug that acts via negative allosteric modulation of the α7-nicotinic acetylcholine receptor,[1] by Bionomics Limited. It is currently being investigated for the treatment of post traumatic stress disorder.[2] The drug has demonstrated clinically significant anxiety reduction in both animal models and in Phase I trials.[3]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
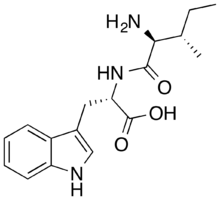
| Formula | C17H23N3O3 |
| Molar mass | 317.389 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
It appears to be devoid of significant sedation or memory-impairing side effects, as well as lacking addictive potential in rat discriminatory models.[4]
Phase I trials have shown no serious side effects.
Bionomics previously licensed it to Ironwood Pharmaceuticals in January 2012, where it was known as IW-2143. In December 2012, IW-2143 begun undergoing phase I clinical trials in the United States,[5] but in November 2014, was released back to Bionomics in a mutual agreement.[6] Bionomics will now continue development and clinical testing, with Ironwood receiving a royalty for their work done.
As of April 2015, BNC210 is in phase II clinical trials.[7] The estimated study completion date is September 2018. [2]
Study Details
The clinical trial is currently testing twice daily oral dosages of 600 mg, 300 mg, and 150 mg, for 12 weeks. [2] The primary objective of the trial is for the treatment of PTSD with a secondary objective for the effectiveness in treating anxiety disorders and depression. [2]
References
- http://www.bionomics.com.au/upload/investors/asx-announcements/4736/ASX695%20%20BNC210%20Phase%201b%20initiation.pdf
- Clinicaltrials.gov. "www.clinicaltrials.gov" (HTML). Clinicaltrials.gov. Retrieved 2018-01-04.
- "Bionomics - Pipeline". Retrieved 2010-11-09.
Bionomics has discovered a novel compound, BNC210, that offers dramatic competitive advantages over existing treatments
- O'Connor, S.; Andriambeloson, E.; Huyard, B.; Wagner, S.; Sleebs, B.; Quasi, N.; Bui, C.; Street, I. "BNC210: A Novel Compound with Potent Anxiolytic Activity" (PDF). Bionomics Limited. Retrieved 2010-11-09.
By applying a targeted medicinal chemistry strategy beginning from a compound cited in the literature, Bionomics has developed BNC210
- "Archived copy". Archived from the original on 2014-05-04. Retrieved 2013-02-01.CS1 maint: archived copy as title (link)
- http://www.biotechdaily.com.au/media/backissues/2014/11%20Nov/BD%20Biotech%20Daily%20Nov%2011.pdf
- Bionomics Limited. "www.prnewswire.com" (HTML). PRNewswire. Retrieved 2015-06-10.