1-Propanol
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Propan-1-ol[1] | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol) |
|||
| 3DMet | B00883 | ||
| 1098242 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.679 | ||
| EC Number | 200-746-9 | ||
| 25616 | |||
| KEGG | |||
| MeSH | 1-Propanol | ||
PubChem CID |
|||
| RTECS number | UH8225000 | ||
| UNII | |||
| UN number | 1274 | ||
| |||
| |||
| Properties | |||
| C3H8O | |||
| Molar mass | 60.10 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Odor | mild, alcohol-like[2] | ||
| Density | 0.803 g/mL | ||
| Melting point | −126 °C; −195 °F; 147 K | ||
| Boiling point | 97 to 98 °C; 206 to 208 °F; 370 to 371 K | ||
| miscible | |||
| log P | 0.329 | ||
| Vapor pressure | 1.99 kPa (at 20 °C) | ||
| Acidity (pKa) | 16 | ||
| Basicity (pKb) | −2 | ||
| -45.176·10−6 cm3/mol | |||
Refractive index (nD) |
1.387 | ||
| Viscosity | 1.959 mPa·s (at 25 °C) [3] | ||
| 1.68 D | |||
| Thermochemistry | |||
Heat capacity (C) |
143.96 J K−1 mol−1 | ||
Std molar entropy (S |
192.8 J K−1 mol−1 | ||
Std enthalpy of formation (ΔfH |
−302.79–−302.29 kJ mol−1 | ||
Std enthalpy of combustion (ΔcH |
−2.02156–−2.02106 MJ mol−1 | ||
| Pharmacology | |||
| D08AX03 (WHO) | |||
| Hazards | |||
| GHS pictograms |  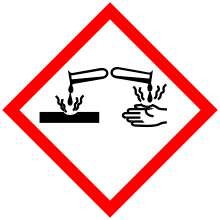  | ||
| GHS signal word | DANGER | ||
| H225, H318, H336 | |||
| P210, P261, P280, P305+351+338 | |||
| NFPA 704 | |||
| Flash point | 22 °C (72 °F; 295 K) | ||
| 371 °C (700 °F; 644 K) | |||
| Explosive limits | 2.2% - 13.7%[2] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
2800 mg/kg (rabbit, oral) 6800 mg/kg (mouse, oral) 1870 mg/kg (rat, oral)[4] | ||
| US health exposure limits (NIOSH): | |||
PEL (Permissible) |
TWA 200 ppm (500 mg/m3)[2] | ||
REL (Recommended) |
TWA 200 ppm (500 mg/m3) ST 250 ppm (625 mg/m3) [skin][2] | ||
IDLH (Immediate danger) |
800 ppm[2] | ||
| Related compounds | |||
Related compounds |
Propane Isopropyl alcohol Propanamine Ethanol Butanol | ||
| Supplementary data page | |||
| Refractive index (n), Dielectric constant (εr), etc. | |||
Thermodynamic data |
Phase behaviour solid–liquid–gas | ||
| UV, IR, NMR, MS | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
1-Propanol is a primary alcohol with the formula CH3CH2CH2OH (sometimes represented as PrOH or n-PrOH). This colorless liquid is also known as propan-1-ol, 1-propyl alcohol, n-propyl alcohol, and n-propanol. It is an isomer of 2-propanol (propan-2-ol, isopropyl alcohol, isopropanol). It is formed naturally in small amounts during many fermentation processes and used as a solvent in the pharmaceutical industry mainly for resins and cellulose esters.
Chemical properties
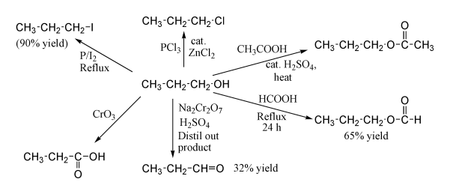
1-Propanol shows the normal reactions of a primary alcohol. Thus it can be converted to alkyl halides; for example red phosphorus and iodine produce n-propyl iodide in 80% yield, while PCl3 with catalytic ZnCl2 gives n-propyl chloride. Reaction with acetic acid in the presence of an H2SO4 catalyst under Fischer esterification conditions gives propyl acetate, while refluxing propanol overnight with formic acid alone can produce propyl formate in 65% yield. Oxidation of 1-propanol with Na2Cr2O7 and H2SO4 gives only a 36% yield of propionaldehyde, and therefore for this type of reaction higher yielding methods using PCC or the Swern oxidation are recommended. Oxidation with chromic acid yields propionic acid.
Preparation
1-Propanol is manufactured by catalytic hydrogenation of propionaldehyde. The propionaldehyde is itself produced via the oxo process, by hydroformylation of ethylene using carbon monoxide and hydrogen in the presence of a catalyst such as cobalt octacarbonyl or a rhodium complex.[5]
- H2C=CH2 + CO + H2 → CH3CH2CH=O
- CH3CH2CH=O + H2 → CH3CH2CH2OH
A traditional laboratory preparation of 1-propanol involves treating n-propyl iodide with moist Ag2O.
1-Propanol was discovered in 1853 by Gustave C. B. Chancel, who obtained it by fractional distillation of fusel oil. Indeed, 1-propanol is a major constituent of fusel oil, a by-product formed from certain amino acids when potatoes or grains are fermented to produce ethanol. This process is no longer a significant source of 1-propanol.
Safety
1-Propanol is thought to be similar to ethanol in its effects on the human body, but 2-4 times more potent. Oral LD50 in rats is 1870 mg/kg (compared to 7060 mg/kg for ethanol). It is metabolized into propionic acid. Effects include alcoholic intoxication and high anion gap metabolic acidosis. As of 2011, only one case of lethal 1-propanol poisoning was reported.[6]
Inhalation
Although this method is rare, it does exist. Propanol might be much more convenient than ethanol for inhalation because of its potency with nebulizers.
Propanol as fuel
1-propanol has high octane numbers and it is suitable for engine fuel usage. However, the production of propanol has been too expensive to make it a common fuel. The research octane number (RON) of propanol is 118 and anti-knock index (AKI) is 108.[7]
References
- ↑ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 61. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- 1 2 3 4 5 "NIOSH Pocket Guide to Chemical Hazards #0533". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Pal, Amalendu; Gaba, Rekha (2008). "Volumetric, acoustic, and viscometric studies of molecular interactions in binary mixtures of dipropylene glycol dimethyl ether with 1-alkanols at 298.15 K". The Journal of Chemical Thermodynamics. 40 (5): 818–828. doi:10.1016/j.jct.2008.01.008.
- ↑ "n-Propyl alcohol". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ↑ Anthony J. Papa "Propanols" in Ullmann’s Encyclopedia of Industrial Chemistry 2011, Wiley-VCH, Weinheim. doi:10.1002/14356007.a22_173.pub2
- ↑ "N-PROPANOL Health-Base Assessment and Recommendation for HEAC" (PDF).
- ↑ "Bioalcohols". Biofuel.org.uk. Retrieved 2014-04-16.
Further reading
- Furniss, B. S.; Hannaford, A. J.; Smith, P. W. G.; Tatchell, A. R. (1989), Vogel's Textbook of Practical Organic Chemistry (5th ed.), Harlow: Longman, ISBN 0-582-46236-3
- Lide, David R., ed. (2006-06-26). CRC Handbook of Chemistry and Physics, 87th Edition (87 ed.). TF-CRC. ISBN 0-8493-0487-3.
- Maryadele J. O'Neil, ed. (2006-11-03). The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (14 ed.). Merck. ISBN 0-911910-00-X.
- Perkin, W. H.; Kipping, F. S (1922). Organic Chemistry. London: W. & R. Chambers. ISBN 0-08-022354-0.