Saturated and unsaturated compounds
In organic chemistry, a saturated compound is a chemical compound that has single bonds.
Saturated compounds
| Saturated compounds | |
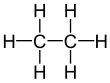
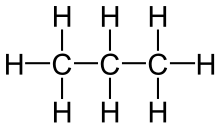
| Ethane | Propane |
 |
 |
| 1-Octanol | |
| Saturated fatty acids | |
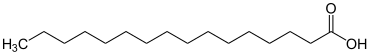 | |
Saturated compounds only possess carbon-carbon single bonds.
Properties
Saturated compounds are generally more stable and less reactive than unsaturated compounds since no steric hindrances and no major polarity differences occur. In addition, they have very low melting and boiling points relative to their molar mass.
Examples
Saturated hydrocarbons
Saturated alcohols, aldehydes, ketones, carboxylic acids and esters
Unsaturated compounds
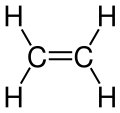
| Unsaturated compounds | |
|---|---|
 |
|
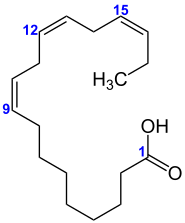 | |
Unsaturated compounds are organic-chemical compounds whose molecular structure contains one or more carbon-carbon double bonds or triple bonds. These can also be conjugated. Examples are the unsaturated fatty acids or unsaturated hydrocarbons (alkenes and alkynes). Many natural products are unsaturated compounds.
History
The term unsaturated compounds, originally unsaturated hydrocarbons, derives from their ability to carry out typical addition reactions which are not possible with saturated compounds such as alkanes:
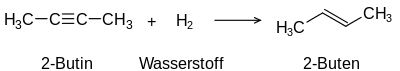
Addition of hydrogen to an alkyne
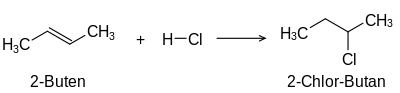
Addition of hydrogen chloride (chlorination)
Here, the only formally unsaturated aromatics differ from the other unsaturated compounds: due to the high stability of the aromatic system, aromatics do not carry out the typical addition reactions or only under extreme conditions such as high temperature or high pressure. Instead, substitution reactions are preferred.
Properties
Unsaturated compounds are generally more reactive than saturated compounds. Triglycerides (rapeseed oil, linseed oil, olive oil, etc.) with a high content of unsaturated fatty acid residues turn more rapidly rancid than those with a high proportion of saturated fatty acid residues ,as for example coconut fat.
In a long chain of carbons, such as a fatty acid, a double or triple bond will cause a kink in the chain. These kinks have macro-structural implications. Unsaturated fats tend to be liquid at room temperature, rather than solid, as the kinks in the chain prevent the molecules from packing closely together to form a solid. These fats are called oils and are present in fish and plants.
In other unsaturated hydrocarbons, the double bond between two carbons prevents rotation of the atoms about the bond, locking them into specific structural formations. When attached atoms occupy similar positions on each carbon, they are referred to as "cis", and when they are on opposite sides, they are called "trans". Most natural hydrocarbons exist in the cis state, but artificially manufactured hydrocarbons are trans. The body lacks the enzymes to properly break down the trans configuration. This is why trans fats are viewed as dangerous and unhealthy, as they tend to build up. Unsaturated compounds of the two formations are classified as geometric isomers of one another.
The amount of unsaturation of a fatty acid can be determined by finding its iodine number.
Examples
Unsaturated hydrocarbons
Unsaturated alcohols, aldehydes, ketones, carboxylic acids and esters
- Ascorbic acid, acetylformoin, bombycol, retinol
- Citronellal, crotonaldehyde, E160e, furfural, retinal, saffronal
- Abscisic acid, anthrone, cyclohexenone
(the solution which contains as much solute as it can dissolveat thesaid temperature in the presence of undissolved solute particles is called a "SATURATED SOLUTION")