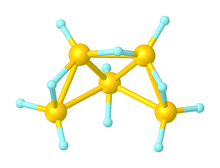
Pentaborane(11)
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.038.447 |
| |
| |
| Properties | |
| B5H11 | |
| Molar mass | 65.14 g·mol−1 |
| Melting point | −123 °C (−189 °F; 150 K) |
| Boiling point | 63 °C (145 °F; 336 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Pentaborane(11) is inorganic compound with the chemical formula B5H11. It is an obscure boron hydride cluster, especially relative to the heavily studied pentaborane(9) (B5H9). With two more hydrogen atoms than nido-pentaborane(9), pentaborane(11) is classified as an arachno cluster.[1]
Synthesis
Like many boron hydride clusters, pentaborane(11) was originally obtained from the pyrolysis of diborane. A more systematic synthesis entails treatment of [B4H9]- with boron tribromide. The Lewis acid abstracts hydride to give unstable B4H8, the precursor to B5H11:[2]
- [B4H9]- + BBr3 → B4H8 + HBBr3-
- 2 B4H8 → B5H11 + "B3H5"
References
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0-08-037941-9.
- ↑ Toft, Mark A.; Leach, J. B.; Himpsl, Francis L.; Shore, Sheldon G. (1982). "New, Systematic Syntheses of Boron Hydrides via Hydride ion Abstraction Reactions: Preparation of B2H6, B4H10, B5H11, and B10H14". Inorganic Chemistry. 21: 1952–7. doi:10.1021/ic00135a048.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.