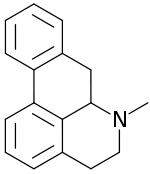
Aporphine
 | |
| Names | |
|---|---|
| IUPAC name
6-Methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinoline | |
| Identifiers | |
3D model (JSmol) |
|
| 192257 | |
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C17H17N | |
| Molar mass | 235.33 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Aporphine is an alkaloid that forms the core of a class of quinoline alkaloids. It can exist in either of two enantiomeric forms, (R)-aporphine and (S)-aporphine.
Many different derivatives have been isolated from plants.[1] For example, many water-lilies (Nymphaea species) produce aporphine alkaloids such as nymphaeine, nymphaline, nupharine, alfa- and beta-nupharidine.[2]
In vitro tests of some aporphine derivatives isolated from Cassytha filiformis, namely actinodaphnine, cassythine, and dicentrine, showed antiparasitic activity against Trypanosoma brucei. Investigation of possible mechanisms revealed that the compounds bind to DNA and act as intercalating agents, besides inhibiting topoisomerase activity.[3]
(R)-Aporphine is a dopamine receptor D1 antagonist with a Ki of 717 nM[4] and a dopamine receptor D2 antagonist with a Ki of 527 nM.[5] Aporphine and its related alkaloids bulbocapnine, boldine, glaucine, and corytuberine are antipsychotic, exert naloxone-reversible antinociceptive activity, and with the exception of corytuberine are anticonvulsant.[6] Some derivatives of aporphine such as (S)-(+)-N-propylnorapomorphine have potential as low side effect profile antipsychotics. (S)-(+)-N-Propylnorapomorphine is highly selective for meso-limbic dopaminergic tracts and function as efficacious partial agonists, with no elevation in prolactin.[7]
See also
References
- ↑ Stévigny, C.; Bailly, C.; Quetin-Leclercq, J. (2005). "Cytotoxic and antitumor potentialities of aporphinoid alkaloids". Current Medicinal Chemistry. Anti-cancer Agents. 5 (2): 173–182. doi:10.2174/1568011053174864. hdl:2078.1/10136. PMID 15777224.
- ↑ Oliver-Bever B., 1983, Medicinal plants in tropical West Africa II. Plants acting on the nervous system, Journal of Ethnopharmacology, vol. 7, pp. 1-93.
- ↑ Hoet, S; Stévigny, C; Block, S; Opperdoes, F; Colson, P; Baldeyrou, B; Lansiaux, A; Bailly, C; Quetin-Leclercq, J (2004). "Alkaloids from Cassytha filiformisand Related Aporphines: Antitrypanosomal Activity, Cytotoxicity, and Interaction with DNA and Topoisomerases". Planta Medica. 70 (5): 407–13. doi:10.1055/s-2004-818967. PMID 15124084.
- ↑ Hedberg MH, Linnanen T, Jansen JM, et al. (August 1996). "11-substituted (R)-aporphines: synthesis, pharmacology, and modeling of D2A and 5-HT1A receptor interactions". Journal of Medicinal Chemistry. 39 (18): 3503–13. doi:10.1021/jm960189i. PMID 8784448.
- ↑ Linnanen T, Brisander M, Unelius L, et al. (April 2001). "Atropisomeric derivatives of 2',6'-disubstituted (R)-11-phenylaporphine: selective serotonin 5-HT(7) receptor antagonists". Journal of Medicinal Chemistry. 44 (9): 1337–40. doi:10.1021/jm0108505. PMID 11311055.
- ↑ Zetler G (1988). "Neuroleptic-like, anticonvulsant and antinociceptive effects of aporphine alkaloids: bulbocapnine, corytuberine, boldine and glaucine". Archives Internationales de Pharmacodynamie et de Thérapie. 296: 255–81. PMID 2907279.
- ↑ Baldessarini RJ, Campbell A, Ben-Jonathan N, Ellingboe J, Zong R, Neumeyer JL (August 1994). "Effects of aporphine isomers on rat prolactin". Neuroscience Letters. 176 (2): 269–71. doi:10.1016/0304-3940(94)90098-1. PMID 7830962.