JWH-250
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C22H25NO2 |
| Molar mass | 335.439 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
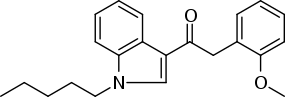
JWH-250 or (1-pentyl-3-(2-methoxyphenylacetyl)indole) is an analgesic chemical from the phenylacetylindole family that acts as a cannabinoid agonist at both the CB1 and CB2 receptors, with a Ki of 11 nM at CB1 and 33 nM at CB2. Unlike many of the older JWH series compounds, this compound does not have a naphthalene ring, instead occupying this position with a 2'-methoxy-phenylacetyl group, making JWH-250 a representative member of a new class of cannabinoid ligands.[2] Other 2'-substituted analogues such as the methyl, chloro and bromo compounds are also active and somewhat more potent.[3][4]
History
JWH-250 was discovered by, and named after the researcher Dr. John W. Huffman. He created JWH-250 and a number of other compounds to research the structure and function of the endocannabinoid system of mammals. Samples of JWH-250 were first identified in May 2009 by the German Federal Criminal Police, as an ingredient in new generation "herbal smoking blends" that had been released since the banning of the original ingredients (C8)-CP 47,497 and JWH-018.[5] An ELISA immunoassay technique for detecting JWH-250 in urine has been reported.[6]
Legal Status
Australia
JWH-250 is considered a Schedule 9 prohibited substance in Australia under the Poisons Standard (October 2015).[7] A Schedule 9 substance is a substance which may be abused or misused, the manufacture, possession, sale or use of which should be prohibited by law except when required for medical or scientific research, or for analytical, teaching or training purposes with approval of Commonwealth and/or State or Territory Health Authorities.[7]
See also
References
- ↑ Legal article in Latvian (www.likumi.lv)
- ↑ Huffman, JW; et al. (2005). "1-Pentyl-3-phenylacetylindoles, a new class of cannabimimetic indoles". Bioorganic & Medicinal Chemistry Letters. 15 (18): 4110–3. doi:10.1016/j.bmcl.2005.06.008. PMID 16005223.
- ↑ Manera, C; Tuccinardi, T; Martinelli, A (2008). "Indoles and related compounds as cannabinoid ligands". Mini Reviews in Medicinal Chemistry. 8 (4): 370–87. doi:10.2174/138955708783955935. PMID 18473928.
- ↑ The Cannabinoid Receptors. Edited by Patricia H Reggio. Humana Press 2009. ISBN 978-1-58829-712-9
- ↑ Understanding the ‘Spice’ phenomenon. EMCDDA, Lisbon, November 2009
- ↑ Arntson et al (2013) http://jat.oxfordjournals.org/content/37/5/284.abstract
- 1 2 Poisons Standard October 2015 https://www.comlaw.gov.au/Details/F2015L01534