Genetic engineering
| Part of a series on |
| Genetics |
|---|
 |
| Key components |
| History and topics |
| Research |
|
| Personalized medicine |
| Personalized medicine |
|
Genetic engineering, also called genetic modification or genetic manipulation, is the direct manipulation of an organism's genes using biotechnology. It is a set of technologies used to change the genetic makeup of cells, including the transfer of genes within and across species boundaries to produce improved or novel organisms. New DNA is obtained by either isolating and copying the genetic material of interest using recombinant DNA methods or by artificially synthesising the DNA. A construct is usually created and used to insert this DNA into the host organism. The first recombinant DNA molecule was made by Paul Berg in 1972 by combining DNA from the monkey virus SV40 with the lambda virus. As well as inserting genes, the process can be used to remove, or "knock out", genes. The new DNA can be inserted randomly, or targeted to a specific part of the genome.
An organism that is generated through genetic engineering is considered to be genetically modified (GM) and the resulting entity is a genetically modified organism (GMO). The first GMO was a bacterium generated by Herbert Boyer and Stanley Cohen in 1973. Rudolf Jaenisch created the first GM animal when he inserted foreign DNA into a mouse in 1974. The first company to focus on genetic engineering, Genentech, was founded in 1976 and started the production of human proteins. Genetically engineered human insulin was produced in 1978 and insulin-producing bacteria were commercialised in 1982. Genetically modified food has been sold since 1994, with the release of the Flavr Savr tomato. The Flavr Savr was engineered to have a longer shelf life, but most current GM crops are modified to increase resistance to insects and herbicides. GloFish, the first GMO designed as a pet, was sold in the United States in December 2003. In 2016 salmon modified with a growth hormone were sold.
Genetic engineering has been applied in numerous fields including research, medicine, industrial biotechnology and agriculture. In research GMOs are used to study gene function and expression through loss of function, gain of function, tracking and expression experiments. By knocking out genes responsible for certain conditions it is possible to create animal model organisms of human diseases. As well as producing hormones, vaccines and other drugs genetic engineering has the potential to cure genetic diseases through gene therapy. The same techniques that are used to produce drugs can also have industrial applications such as producing enzymes for laundry detergent, cheeses and other products.
The rise of commercialised genetically modified crops has provided economic benefit to farmers in many different countries, but has also been the source of most of the controversy surrounding the technology. This has been present since its early use, the first field trials were destroyed by anti-GM activists. Although there is a scientific consensus that currently available food derived from GM crops poses no greater risk to human health than conventional food, GM food safety is a leading concern with critics. Gene flow, impact on non-target organisms, control of the food supply and intellectual property rights have also been raised as potential issues. These concerns have led to the development of a regulatory framework, which started in 1975. It has led to an international treaty, the Cartagena Protocol on Biosafety, that was adopted in 2000. Individual countries have developed their own regulatory systems regarding GMOs, with the most marked differences occurring between the USA and Europe.
Overview
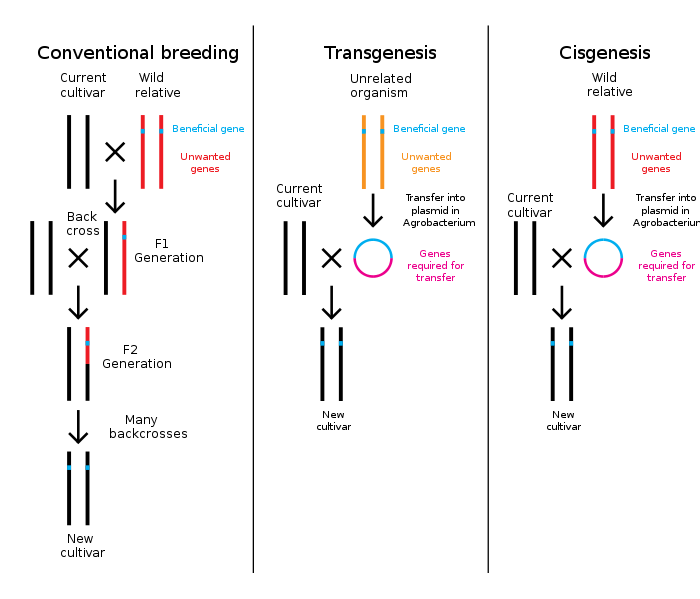
Genetic engineering is a process that alters the genetic structure of an organism by either removing or introducing DNA. Unlike traditionally animal and plant breeding, which involves doing multiple crosses and then selecting for the organism with the desired phenotype, genetic engineering takes the gene directly from one organism and inserts it in the other. This is much faster, can be used to insert any genes from any organism (even ones from different domains) and prevents other undesirable genes from also being added.[3]
Genetic engineering could potentially fix severe genetic disorders in humans by replacing the defective gene with a functioning one.[4] It is an important tool in research that allows the function of specific genes to be studied.[5] Drugs, vaccines and other products have been harvested from organisms engineered to produce them.[6] Crops have been developed that aid food security by increasing yield, nutritional value and tolerance to environmental stresses.[7]
The DNA can be introduced directly into the host organism or into a cell that is then fused or hybridised with the host.[8] This relies on recombinant nucleic acid techniques to form new combinations of heritable genetic material followed by the incorporation of that material either indirectly through a vector system or directly through micro-injection, macro-injection or micro-encapsulation.[9]
Genetic engineering does not normally include traditional breeding, in vitro fertilisation, induction of polyploidy, mutagenesis and cell fusion techniques that do not use recombinant nucleic acids or a genetically modified organism in the process.[8] However, some broad definitions of genetic engineering include selective breeding.[9] Cloning and stem cell research, although not considered genetic engineering,[10] are closely related and genetic engineering can be used within them.[11] Synthetic biology is an emerging discipline that takes genetic engineering a step further by introducing artificially synthesised material into an organism.[12]
Plants, animals or micro organisms that have been changed through genetic engineering are termed genetically modified organisms or GMOs.[13] If genetic material from another species is added to the host, the resulting organism is called transgenic. If genetic material from the same species or a species that can naturally breed with the host is used the resulting organism is called cisgenic.[14] If genetic engineering is used to remove genetic material from the target organism the resulting organism is termed a knockout organism.[15] In Europe genetic modification is synonymous with genetic engineering while within the United States of America and Canada genetic modification can also be used to refer to more conventional breeding methods.[16][17][18]
History
Humans have altered the genomes of species for thousands of years through selective breeding, or artificial selection[19]:1[20]:1 as contrasted with natural selection, and more recently through mutagenesis. Genetic engineering as the direct manipulation of DNA by humans outside breeding and mutations has only existed since the 1970s. The term "genetic engineering" was first coined by Jack Williamson in his science fiction novel Dragon's Island, published in 1951[21] – one year before DNA's role in heredity was confirmed by Alfred Hershey and Martha Chase,[22] and two years before James Watson and Francis Crick showed that the DNA molecule has a double-helix structure – though the general concept of direct genetic manipulation was explored in rudimentary form in Stanley G. Weinbaum's 1936 science fiction story Proteus Island.[23][24]

In 1972, Paul Berg created the first recombinant DNA molecules by combining DNA from the monkey virus SV40 with that of the lambda virus.[25] In 1973 Herbert Boyer and Stanley Cohen created the first transgenic organism by inserting antibiotic resistance genes into the plasmid of an Escherichia coli bacterium.[26][27] A year later Rudolf Jaenisch created a transgenic mouse by introducing foreign DNA into its embryo, making it the world’s first transgenic animal.[28] These achievements led to concerns in the scientific community about potential risks from genetic engineering, which were first discussed in depth at the Asilomar Conference in 1975. One of the main recommendations from this meeting was that government oversight of recombinant DNA research should be established until the technology was deemed safe.[29][30]
In 1976 Genentech, the first genetic engineering company, was founded by Herbert Boyer and Robert Swanson and a year later the company produced a human protein (somatostatin) in E.coli. Genentech announced the production of genetically engineered human insulin in 1978.[31] In 1980, the U.S. Supreme Court in the Diamond v. Chakrabarty case ruled that genetically altered life could be patented.[32] The insulin produced by bacteria was approved for release by the Food and Drug Administration (FDA) in 1982.[33]
In 1983, a biotech company, Advanced Genetic Sciences (AGS) applied for U.S. government authorisation to perform field tests with the ice-minus strain of Pseudomonas syringae to protect crops from frost, but environmental groups and protestors delayed the field tests for four years with legal challenges.[34] In 1987, the ice-minus strain of P. syringae became the first genetically modified organism (GMO) to be released into the environment[35] when a strawberry field and a potato field in California were sprayed with it.[36] Both test fields were attacked by activist groups the night before the tests occurred: "The world's first trial site attracted the world's first field trasher".[35]
The first field trials of genetically engineered plants occurred in France and the USA in 1986, tobacco plants were engineered to be resistant to herbicides.[37] The People’s Republic of China was the first country to commercialise transgenic plants, introducing a virus-resistant tobacco in 1992.[38] In 1994 Calgene attained approval to commercially release the first genetically modified food, the Flavr Savr, a tomato engineered to have a longer shelf life.[39] In 1994, the European Union approved tobacco engineered to be resistant to the herbicide bromoxynil, making it the first genetically engineered crop commercialised in Europe.[40] In 1995, Bt Potato was approved safe by the Environmental Protection Agency, after having been approved by the FDA, making it the first pesticide producing crop to be approved in the USA.[41] In 2009 11 transgenic crops were grown commercially in 25 countries, the largest of which by area grown were the USA, Brazil, Argentina, India, Canada, China, Paraguay and South Africa.[42]
In 2010, scientists at the J. Craig Venter Institute created the first synthetic genome and inserted it into an empty bacterial cell. The resulting bacterium, named Mycoplasma laboratorium, could replicate and produce proteins.[43][44] Four years later this was taken a step further when a bacterium was developed that replicated a plasmid containing a unique base pair, creating the first organism engineered to use an expanded genetic alphabet.[45][46] In 2012, Jennifer Doudna and Emmanuelle Charpentier collaborated to develop the CRISPR/Cas9 system,[47][48] a technique which can be used to easily and specifically alter the genome of almost any organism.[49]
Process
Creating a GMO is a multi-step process. Genetic engineers must first choose what gene they wish to insert into the organism. This is driven by what the aim is for the resultant organism and is built on earlier research. Genetic screens can be carried out to determine potential genes and further tests then used to identify the best candidates. The development of microarrays, transcriptomics and genome sequencing has made it much easier to find suitable genes.[50] Luck also plays its part; the round-up ready gene was discovered after scientists noticed a bacterium thriving in the presence of the herbicide.[51]
Gene isolation and cloning
The next step is to isolate the candidate gene. The cell containing the gene is opened and the DNA is purified.[52] The gene is separated by using restriction enzymes to cut the DNA into fragments[53] or polymerase chain reaction (PCR) to amplify up the gene segment.[54] These segments can then be extracted through gel electrophoresis. If the chosen gene or the donor organism's genome has been well studied it may already be accessible from a genetic library. If the DNA sequence is known, but no copies of the gene are available, it can also be artificially synthesised.[55] Once isolated the gene is ligated into a plasmid that is then inserted into a bacterium. The plasmid is replicated when the bacteria divide, ensuring unlimited copies of the gene are available.[56]
Before the gene is inserted into the target organism it must be combined with other genetic elements. These include a promoter and terminator region, which initiate and end transcription. A selectable marker gene is added, which in most cases confers antibiotic resistance, so researchers can easily determine which cells have been successfully transformed. The gene can also be modified at this stage for better expression or effectiveness. These manipulations are carried out using recombinant DNA techniques, such as restriction digests, ligations and molecular cloning.[57]
Inserting DNA into the host genome

There are a number of techniques available for inserting the gene into the host genome. Some bacteria can naturally take up foreign DNA. This ability can be induced in other bacteria via stress (e.g. thermal or electric shock), which increases the cell membrane's permeability to DNA; up-taken DNA can either integrate with the genome or exist as extrachromosomal DNA. DNA is generally inserted into animal cells using microinjection, where it can be injected through the cell's nuclear envelope directly into the nucleus, or through the use of viral vectors.[58]
In plants the DNA is often inserted using Agrobacterium-mediated recombination,[59] taking advantage of the Agrobacteriums T-DNA sequence that allows natural insertion of genetic material into plant cells.[60] Other methods include biolistics, where particles of gold or tungsten are coated with DNA and then shot into young plant cells,[61] and electroporation, which involves using an electric shock to make the cell membrane permeable to plasmid DNA. Due to the damage caused to the cells and DNA the transformation efficiency of biolistics and electroporation is lower than agrobacterial transformation and microinjection.[62]
As only a single cell is transformed with genetic material, the organism must be regenerated from that single cell. In plants this is accomplished through the use of tissue culture.[63][64] In animals it is necessary to ensure that the inserted DNA is present in the embryonic stem cells.[65] Bacteria consist of a single cell and reproduce clonally so regeneration is not necessary. Selectable markers are used to easily differentiate transformed from untransformed cells. These markers are usually present in the transgenic organism, although a number of strategies have been developed that can remove the selectable marker from the mature transgenic plant.[66]
Further testing using PCR, Southern hybridization, and DNA sequencing is conducted to confirm that an organism contains the new gene.[67] These tests can also confirm the chromosomal location and copy number of the inserted gene. The presence of the gene does not guarantee it will be expressed at appropriate levels in the target tissue so methods that look for and measure the gene products (RNA and protein) are also used. These include northern hybridisation, quantitative RT-PCR, Western blot, immunofluorescence, ELISA and phenotypic analysis.[68]
The new genetic material can be inserted randomly within the host genome or targeted to a specific location. The technique of gene targeting uses homologous recombination to make desired changes to a specific endogenous gene. This tends to occur at a relatively low frequency in plants and animals and generally requires the use of selectable markers. The frequency of gene targeting can be greatly enhanced through genome editing. Genome editing uses artificially engineered nucleases that create specific double-stranded breaks at desired locations in the genome, and use the cell’s endogenous mechanisms to repair the induced break by the natural processes of homologous recombination and nonhomologous end-joining. There are four families of engineered nucleases: meganucleases,[69][70] zinc finger nucleases,[71][72] transcription activator-like effector nucleases (TALENs),[73][74] and the Cas9-guideRNA system (adapted from CRISPR).[75][76] TALEN and CRISPR are the two most commonly used and each has its own advantages.[77] TALENs have greater target specificity, while CRISPR is easier to design and more efficient.[77] In addition to enhancing gene targeting, engineered nucleases can be used to introduce mutations at endogenous genes that generate a gene knockout.[78][79]
Applications
Genetic engineering has applications in medicine, research, industry and agriculture and can be used on a wide range of plants, animals and micro organisms. Bacteria, the first organisms to be genetically modified, can have plasmid DNA inserted containing new genes that code for medicines or enzymes that process food and other substrates.[80][81] Plants have been modified for insect protection, herbicide resistance, virus resistance, enhanced nutrition, tolerance to environmental pressures and the production of edible vaccines.[82] Most commercialised GMOs are insect resistant or herbicide tolerant crop plants.[83] Genetically modified animals have been used for research, model animals and the production of agricultural or pharmaceutical products. The genetically modified animals include animals with genes knocked out, increased susceptibility to disease, hormones for extra growth and the ability to express proteins in their milk.[84]
Medicine
Genetic engineering has many applications to medicine that include the manufacturing of drugs, creation of model animals that mimic human conditions and gene therapy. One of the earliest uses of genetic engineering was to mass-produce human insulin in bacteria.[31] This application has now been applied to, human growth hormones, follicle stimulating hormones (for treating infertility), human albumin, monoclonal antibodies, antihemophilic factors, vaccines and many other drugs.[85][86] Mouse hybridomas, cells fused together to create monoclonal antibodies, have been adapted through genetic engineering to create human monoclonal antibodies.[87] In 2017, genetic engineering of chimeric antigen receptors on a patient's own T-cells was approved by the U.S. FDA as a treatment for the cancer acute lymphoblastic leukemia. Genetically engineered viruses are being developed that can still confer immunity, but lack the infectious sequences.[88]
Genetic engineering is also used to create animal models of human diseases. Genetically modified mice are the most common genetically engineered animal model.[89] They have been used to study and model cancer (the oncomouse), obesity, heart disease, diabetes, arthritis, substance abuse, anxiety, aging and Parkinson disease.[90] Potential cures can be tested against these mouse models. Also genetically modified pigs have been bred with the aim of increasing the success of pig to human organ transplantation.[91]
Gene therapy is the genetic engineering of humans, generally by replacing defective genes with effective ones. Clinical research using somatic gene therapy has been conducted with several diseases, including X-linked SCID,[92] chronic lymphocytic leukemia (CLL),[93][94] and Parkinson's disease.[95] In 2012, Alipogene tiparvovec became the first gene therapy treatment to be approved for clinical use.[96][97] In 2015 a virus was used to insert a healthy gene into the skin cells of a boy suffering from a rare skin disease, epidermolysis bullosa, in order to grow, and then graft healthy skin onto 80 percent of the boy's body which was affected by the illness.[98] Germline gene therapy would result in any change being inheritable, which has raised concerns within the scientific community.[99][100] In 2015, CRISPR was used to edit the DNA of non-viable human embryos,[101][102] leading scientists of major world academies to call for a moratorium on inheritable human genome edits.[103] There are also concerns that the technology could be used not just for treatment, but for enhancement, modification or alteration of a human beings' appearance, adaptability, intelligence, character or behavior.[104] The distinction between cure and enhancement can also be difficult to establish.[105]
Researchers are altering the genome of pigs to induce the growth of human organs to be used in transplants. Scientists are creating "gene drives", changing the genomes of mosquitoes to make them immune to malaria, and then spreading the genetically altered mosquitoes throughout the mosquito population in the hopes of eliminating the disease.[106]
Research
Genetic engineering is an important tool for natural scientists. Genes and other genetic information from a wide range of organisms can be inserted into bacteria for storage and modification, creating genetically modified bacteria in the process. Bacteria are cheap, easy to grow, clonal, multiply quickly, relatively easy to transform and can be stored at -80 °C almost indefinitely. Once a gene is isolated it can be stored inside the bacteria providing an unlimited supply for research.[107]
Organisms are genetically engineered to discover the functions of certain genes. This could be the effect on the phenotype of the organism, where the gene is expressed or what other genes it interacts with. These experiments generally involve loss of function, gain of function, tracking and expression.
- Loss of function experiments, such as in a gene knockout experiment, in which an organism is engineered to lack the activity of one or more genes. In a simple knockout a copy of the desired gene has been altered to make it non-functional. Embryonic stem cells incorporate the altered gene, which replaces the already present functional copy. These stem cells are injected into blastocysts, which are implanted into surrogate mothers. This allows the experimenter to analyse the defects caused by this mutation and thereby determine the role of particular genes. It is used especially frequently in developmental biology.[108] When this is done by creating a library of genes with point mutations at every position in the area of interest, or even every position in the whole gene, this is called "scanning mutagenesis". The simplest method, and the first to be used, is "alanine scanning", where every position in turn is mutated to the unreactive amino acid alanine.[109]
- Gain of function experiments, the logical counterpart of knockouts. These are sometimes performed in conjunction with knockout experiments to more finely establish the function of the desired gene. The process is much the same as that in knockout engineering, except that the construct is designed to increase the function of the gene, usually by providing extra copies of the gene or inducing synthesis of the protein more frequently. Gain of function is used to tell whether or not a protein is sufficient for a function, but does not always mean it's required, especially when dealing with genetic or functional redundancy.[108]
- Tracking experiments, which seek to gain information about the localisation and interaction of the desired protein. One way to do this is to replace the wild-type gene with a 'fusion' gene, which is a juxtaposition of the wild-type gene with a reporting element such as green fluorescent protein (GFP) that will allow easy visualisation of the products of the genetic modification. While this is a useful technique, the manipulation can destroy the function of the gene, creating secondary effects and possibly calling into question the results of the experiment. More sophisticated techniques are now in development that can track protein products without mitigating their function, such as the addition of small sequences that will serve as binding motifs to monoclonal antibodies.[108]
- Expression studies aim to discover where and when specific proteins are produced. In these experiments, the DNA sequence before the DNA that codes for a protein, known as a gene's promoter, is reintroduced into an organism with the protein coding region replaced by a reporter gene such as GFP or an enzyme that catalyses the production of a dye. Thus the time and place where a particular protein is produced can be observed. Expression studies can be taken a step further by altering the promoter to find which pieces are crucial for the proper expression of the gene and are actually bound by transcription factor proteins; this process is known as promoter bashing.[110]
Industrial
Organisms can have their cells transformed with a gene coding for a useful protein, such as an enzyme, so that they will overexpress the desired protein. Mass quantities of the protein can then be manufactured by growing the transformed organism in bioreactor equipment using industrial fermentation, and then purifying the protein.[111] Some genes do not work well in bacteria, so yeast, insect cells or mammalians cells can also be used.[112] These techniques are used to produce medicines such as insulin, human growth hormone, and vaccines, supplements such as tryptophan, aid in the production of food (chymosin in cheese making) and fuels.[113] Other applications with genetically engineered bacteria could involve making them perform tasks outside their natural cycle, such as making biofuels,[114] cleaning up oil spills, carbon and other toxic waste[115] and detecting arsenic in drinking water.[116] Certain genetically modified microbes can also be used in biomining and bioremediation, due to their ability to extract heavy metals from their environment and incorporate them into compounds that are more easily recoverable.[117]
In materials science, a genetically modified virus has been used in a research laboratory as a scaffold for assembling a more environmentally friendly lithium-ion battery.[118][119] Bacteria have also been engineered to function as sensors by expressing a fluorescent protein under certain environmental conditions.[120]
Agriculture

One of the best-known and controversial applications of genetic engineering is the creation and use of genetically modified crops or genetically modified livestock to produce genetically modified food. Crops have been developed to increase production, increase tolerance to abiotic stresses, alter the composition of the food, or to produce novel products.[122]
The first crops to be realised commercially on a large scale provided protection from insect pests or tolerance to herbicides. Fungal and virus resistant crops have also been developed or are in development.[123][124] This make the insect and weed management of crops easier and can indirectly increase crop yield.[125][126] GM crops that directly improve yield by accelerating growth or making the plant more hardy (by improving salt, cold or drought tolerance) are also under development.[127] In 2016 Salmon have been genetically modified with growth hormones to reach normal adult size much faster.[128]
GMOs have been developed that modify the quality of produce by increasing the nutritional value or providing more industrially useful qualities or quantities.[127] The Amflora potato produces a more industrially useful blend of starches. Soybeans and canola have been genetically modified to produce more healthy oils.[129][130] The first commercialised GM food was a tomato that had delayed ripening, increasing its shelf life.[131]
Plants and animals have been engineered to produce materials they do not normally make. Pharming uses crops and animals as bioreactors to produce vaccines, drug intermediates, or the drugs themselves; the useful product is purified from the harvest and then used in the standard pharmaceutical production process.[132] Cows and goats have been engineered to express drugs and other proteins in their milk, and in 2009 the FDA approved a drug produced in goat milk.[133][134]
Other applications
Genetic engineering has potential applications in conservation and natural area management. Gene transfer through viral vectors has been proposed as a means of controlling invasive species as well as vaccinating threatened fauna from disease.[135] Transgenic trees have been suggested as a way to confer resistance to pathogens in wild populations.[136] With the increasing risks of maladaptation in organisms as a result of climate change and other perturbations, facilitated adaptation through gene tweaking could be one solution to reducing extinction risks.[137] Applications of genetic engineering in conservation are thus far mostly theoretical and have yet to be put into practice.
Genetic engineering is also being used to create microbial art.[138] Some bacteria have been genetically engineered to create black and white photographs.[139] Novelty items such as lavender-colored carnations,[140] blue roses,[141] and glowing fish[142][143] have also been produced through genetic engineering.
Regulation
The regulation of genetic engineering concerns the approaches taken by governments to assess and manage the risks associated with the development and release of GMOs. The development of a regulatory framework began in 1975, at Asilomar, California.[144] The Asilomar meeting recommended a set of voluntary guidelines regarding the use of recombinant technology.[145] As the technology improved USA established a committee at the Office of Science and Technology,[146] which assigned regulatory approval of GM food to the USDA, FDA and EPA.[147] The Cartagena Protocol on Biosafety, an international treaty that governs the transfer, handling, and use of GMOs,[148] was adopted on 29 January 2000.[149] One hundred and fifty-seven countries are members of the Protocol and many use it as a reference point for their own regulations.[150]
The legal and regulatory status of GM foods varies by country, with some nations banning or restricting them, and others permitting them with widely differing degrees of regulation.[151][152][153][154] Some countries allow the import of GM food with authorisation, but either do not allow its cultivation (Russia, Norway, Israel) or have provisions for cultivation even though no GM products are yet produced (Japan, South Korea). Most countries that do not allow GMO cultivation do permit research.[155] Some of the most marked differences occurring between the USA and Europe. The US policy focuses on the product (not the process), only looks at verifiable scientific risks and uses the concept of substantial equivalence.[156] The European Union by contrast has possibly the most stringent GMO regulations in the world.[157] All GMOs, along with irradiated food, are considered "new food" and subject to extensive, case-by-case, science-based food evaluation by the European Food Safety Authority. The criteria for authorisation fall in four broad categories: "safety," "freedom of choice," "labelling," and "traceability."[158] The level of regulation in other countries that cultivate GMOs lie in between Europe and the United States.
| Region | Regulators | Notes |
|---|---|---|
| USA | USDA, FDA and EPA[147] | |
| Europe | European Food Safety Authority[158] | |
| Canada | Health Canada and the Canadian Food Inspection Agency[159][160] | Regulated products with novel features regardless of method of origin[161][162] |
| Africa | Common Market for Eastern and Southern Africa[163] | Final decision lies with each individual country.[163] |
| China | Office of Agricultural Genetic Engineering Biosafety Administration[164] | |
| India | Institutional Biosafety Committee, Review Committee on Genetic Manipulation and Genetic Engineering Approval Committee[165] | |
| Argentina | National Agricultural Biotechnology Advisory Committee (environmental impact), the National Service of Health and Agrifood Quality (food safety) and the National Agribusiness Direction (effect on trade)[166] | Final decision made by the Secretariat of Agriculture, Livestock, Fishery and Food.[166] |
| Brazil | National Biosafety Technical Commission (environmental and food safety) and the Council of Ministers (commercial and economical issues)[166] | |
| Australia | Office of the Gene Technology Regulator (oversees all GM products), Therapeutic Goods Administration (GM medicines) and Food Standards Australia New Zealand (GM food).[167][168] | The individual state governments can then assess the impact of release on markets and trade and apply further legislation to control approved genetically modified products.[168] |
One of the key issues concerning regulators is whether GM products should be labeled. The European Commission says that mandatory labeling and traceability are needed to allow for informed choice, avoid potential false advertising[169] and facilitate the withdrawal of products if adverse effects on health or the environment are discovered.[170] The American Medical Association[171] and the American Association for the Advancement of Science[172] say that absent scientific evidence of harm even voluntary labeling is misleading and will falsely alarm consumers. Labeling of GMO products in the marketplace is required in 64 countries.[173] Labeling can be mandatory up to a threshold GM content level (which varies between countries) or voluntary. In Canada and the USA labeling of GM food is voluntary,[174] while in Europe all food (including processed food) or feed which contains greater than 0.9% of approved GMOs must be labelled.[157]
Controversy
Critics have objected to the use of genetic engineering on several grounds, that include ethical, ecological and economic concerns. Many of these concerns involve GM crops and whether food produced from them is safe and what impact growing them will have on the environment. These controversies have led to litigation, international trade disputes, and protests, and to restrictive regulation of commercial products in some countries.[175]
Accusations that scientists are "playing God" and other religious issues have been ascribed to the technology from the beginning.[176] Other ethical issues raised include the patenting of life,[177] the use of intellectual property rights,[178] the level of labeling on products,[179][180] control of the food supply[181] and the objectivity of the regulatory process.[182] Although doubts have been raised,[183] economically most studies have found growing GM crops to be beneficial to farmers.[184][185][186]
Gene flow between GM crops and compatible plants, along with increased use of selective herbicides, can increase the risk of "superweeds" developing.[187] Other environmental concerns involve potential impacts on non-target organisms, including soil microbes,[188] and an increase in secondary and resistant insect pests.[189][190] Many of the environmental impacts regarding GM crops may take many years to be understood and are also evident in conventional agriculture practices.[188][191] With the commercialisation of genetically modified fish there are concerns over what the environmental consequences will be if they escape.[192]
There are three main concerns over the safety of genetically modified food: whether they may provoke an allergic reaction; whether the genes could transfer from the food into human cells; and whether the genes not approved for human consumption could outcross to other crops.[193] There is a scientific consensus[194][195][196][197] that currently available food derived from GM crops poses no greater risk to human health than conventional food,[198][199][200][201][202] but that each GM food needs to be tested on a case-by-case basis before introduction.[203][204][205] Nonetheless, members of the public are much less likely than scientists to perceive GM foods as safe.[206][207][208][209]
See also
References
- ↑ "Terms and Acronyms". U.S. Environmental Protection Agency online. Retrieved 16 July 2015.
- ↑ Vert, Michel; Doi, Yoshiharu; Hellwich, Karl-Heinz; Hess, Michael; Hodge, Philip; Kubisa, Przemyslaw; Rinaudo, Marguerite; Schué, François (2012). "Terminology for biorelated polymers and applications (IUPAC Recommendations 2012)" (PDF). Pure and Applied Chemistry. 84 (2): 377–410. doi:10.1351/PAC-REC-10-12-04.
- ↑ "How does GM differ from conventional plant breeding?". royalsociety.org. Retrieved 2017-11-14.
- ↑ Erwin, Edward; Gendin, Sidney; Kleiman, Lowell (2015-12-22). Ethical Issues in Scientific Research: An Anthology. Routledge. p. 338. ISBN 9781134817740.
- ↑ Alexander, D. R. (2003-05-01). "Uses and abuses of genetic engineering". Postgraduate Medical Journal. 79 (931): 249–251. doi:10.1136/pmj.79.931.249. ISSN 0032-5473. PMC 1742694. PMID 12782769.
- ↑ Nielsen, Jens (2013-07-01). "Production of biopharmaceutical proteins by yeast". Bioengineered. 4 (4): 207–211. doi:10.4161/bioe.22856. ISSN 2165-5979. PMC 3728191. PMID 23147168.
- ↑ Qaim, Matin; Kouser, Shahzad (2013-06-05). "Genetically Modified Crops and Food Security". PLoS ONE. 8 (6): e64879. Bibcode:2013PLoSO...864879Q. doi:10.1371/journal.pone.0064879. ISSN 1932-6203. PMC 3674000. PMID 23755155.
- 1 2 The European Parliament and the council of the European Union (12 March 2001). "Directive on the release of genetically modified organisms (GMOs) Directive 2001/18/EC ANNEX I A". Official Journal of the European Communities.
- 1 2 Staff Economic Impacts of Genetically Modified Crops on the Agri-Food Sector; P. 42 Glossary - Term and Definitions Archived 14 May 2013 at the Wayback Machine. The European Commission Directorate-General for Agriculture, "Genetic engineering: The manipulation of an organism's genetic endowment by introducing or eliminating specific genes through modern molecular biology techniques. A broad definition of genetic engineering also includes selective breeding and other means of artificial selection.", Retrieved 5 November 2012
- ↑ Van Eenennaam, Alison. "Is Livestock Cloning Another Form of Genetic Engineering?" (PDF). agbiotech. Archived from the original (PDF) on 11 May 2011.
- ↑ Suter, David M.; Dubois-Dauphin, Michel; Krause, Karl-Heinz (2006). "Genetic engineering of embryonic stem cells" (PDF). Swiss Med Wkly. 136 (27–28): 413–415. PMID 16897894. Archived from the original (PDF) on 7 July 2011.
- ↑ Andrianantoandro, Ernesto; Basu, Subhayu; Kariga, David K.; Weiss, Ron (16 May 2006). "Synthetic biology: new engineering rules for an emerging discipline". Molecular Systems Biology. 2 (2006.0028): 2006.0028. doi:10.1038/msb4100073. PMC 1681505. PMID 16738572.
- ↑ "What is genetic modification (GM)?". CSIRO.
- ↑ Jacobsen, E.; Schouten, H. J. (2008). "Cisgenesis, a New Tool for Traditional Plant Breeding, Should be Exempted from the Regulation on Genetically Modified Organisms in a Step by Step Approach". Potato Research. 51: 75–88. doi:10.1007/s11540-008-9097-y.
- ↑ Capecchi, Mario R. (2001). "Generating mice with targeted mutations". Nature Medicine. 7 (10): 1086–90. doi:10.1038/nm1001-1086. PMID 11590420.
- ↑ Staff Biotechnology - Glossary of Agricultural Biotechnology Terms Archived 30 August 2014 at the Wayback Machine. United States Department of Agriculture, "Genetic modification: The production of heritable improvements in plants or animals for specific uses, via either genetic engineering or other more traditional methods. Some countries other than the United States use this term to refer specifically to genetic engineering.", Retrieved 5 November 2012
- ↑ Maryanski, James H. (19 October 1999). "Genetically Engineered Foods". Center for Food Safety and Applied Nutrition at the Food and Drug Administration.
- ↑ Staff (28 November 2005) Health Canada - The Regulation of Genetically Modified Food Glossary definition of Genetically Modified: "An organism, such as a plant, animal or bacterium, is considered genetically modified if its genetic material has been altered through any method, including conventional breeding. A 'GMO' is a genetically modified organism.", Retrieved 5 November 2012
- ↑ Root, Clive (2007). Domestication. Greenwood Publishing Groups.
- ↑ Zohary, Daniel; Hopf, Maria; Weiss, Ehud (2012). Domestication of Plants in the Old World: The origin and spread of plants in the old world. Oxford University Press.
- ↑ Stableford, Brian M. (2004). Historical dictionary of science fiction literature. p. 133. ISBN 9780810849389.
- ↑ A, Hershey; Chase, M. (1952). "Independent functions of viral protein and nucleic acid in growth of bacteriophage" (PDF). J Gen Physiol. 36 (1): 39–56. doi:10.1085/jgp.36.1.39. PMC 2147348. PMID 12981234.
- ↑ "Genetic Engineering". Encyclopedia of Science Fiction. 2 April 2015.
- ↑ Shiv Kant Prasad, Ajay Dash (2008). Modern Concepts in Nanotechnology, Volume 5. Discovery Publishing House. ISBN 9788183562966.
- ↑ Jackson, DA; Symons, RH; Berg, P (1 October 1972). "Biochemical Method for Inserting New Genetic Information into DNA of Simian Virus 40: Circular SV40 DNA Molecules Containing Lambda Phage Genes and the Galactose Operon of Escherichia coli". PNAS. 69 (10): 2904–2909. Bibcode:1972PNAS...69.2904J. doi:10.1073/pnas.69.10.2904. PMC 389671. PMID 4342968.
- ↑ Arnold, Paul (2009). "History of Genetics: Genetic Engineering Timeline".
- ↑ Cohen, Stanley N.; Chang, Annie C. Y. (1 May 1973). "Recircularization and Autonomous Replication of a Sheared R-Factor DNA Segment in Escherichia coli Transformants". PNAS. 70: 1293–1297. Bibcode:1973PNAS...70.1293C. doi:10.1073/pnas.70.5.1293. PMC 433482. Retrieved 17 July 2010.
- ↑ Jaenisch, R. and Mintz, B. (1974 ) Simian virus 40 DNA sequences in DNA of healthy adult mice derived from preimplantation blastocysts injected with viral DNA. Proc. Natl. Acad. 71(4) 1250–1254
- ↑ Berg P; Baltimore, D; Brenner, S; Roblin, RO; Singer, MF; et al. (1975). "Summary statement of the Asilomar Conference on recombinant DNA molecules" (PDF). Proc. Natl. Acad. Sci. U.S.A. 72 (6): 1981–4. Bibcode:1975PNAS...72.1981B. doi:10.1073/pnas.72.6.1981. PMC 432675. PMID 806076.
- ↑ NIH Guidelines for research involving recombinant DNA molecules Archived 10 September 2012 at the Wayback Machine.
- 1 2 Goeddel, David; Kleid, Dennis G.; Bolivar, Francisco; Heyneker, Herbert L.; Yansura, Daniel G.; Crea, Roberto; Hirose, Tadaaki; Kraszewski, Adam; Itakura, Keiichi; Riggs, Arthur D. (January 1979). "Expression in Escherichia coli of chemically synthesized genes for human insulin" (PDF). PNAS. 76 (1): 106–110. Bibcode:1979PNAS...76..106G. doi:10.1073/pnas.76.1.106. PMC 382885. PMID 85300.
- ↑ US Supreme Court Cases from Justia & Oyez (16 June 1980). "Diamond V Chakrabarty". 447 (303). Supreme.justia.com. Retrieved 17 July 2010.
- ↑ "Artificial Genes". TIME. 15 November 1982. Retrieved 17 July 2010.
- ↑ Bratspies, Rebecca (2007). "Some Thoughts on the American Approach to Regulating Genetically Modified Organisms". Kansas Journal of Law & Public Policy. 16 (3): 101–131. SSRN 1017832.
- 1 2 BBC News 14 June 2002 GM crops: A bitter harvest?
- ↑ Thomas H. Maugh II for the Los Angeles Times. 9 June 1987. Altered Bacterium Does Its Job : Frost Failed to Damage Sprayed Test Crop, Company Says
- ↑ James, Clive (1996). "Global Review of the Field Testing and Commercialization of Transgenic Plants: 1986 to 1995" (PDF). The International Service for the Acquisition of Agri-biotech Applications. Retrieved 17 July 2010.
- ↑ James, Clive (1997). "Global Status of Transgenic Crops in 1997" (PDF). ISAAA Briefs No. 5.: 31.
- ↑ Bruening, G.; Lyons, J.M. (2000). "The case of the FLAVR SAVR tomato". California Agriculture. 54 (4): 6–7. doi:10.3733/ca.v054n04p6.
- ↑ MacKenzie, Debora (18 June 1994). "Transgenic tobacco is European first". New Scientist.
- ↑ Genetically Altered Potato Ok'd For Crops Lawrence Journal-World - 6 May 1995
- ↑ Global Status of Commercialized Biotech/GM Crops: 2009 ISAAA Brief 41-2009, 23 February 2010. Retrieved 10 August 2010
- ↑ Pennisi, Elizabeth (2010-05-21). "Synthetic Genome Brings New Life to Bacterium". Science. 328 (5981): 958–959. doi:10.1126/science.328.5981.958. PMID 20488994.
- ↑ Gibson, D. G.; Glass, J. I.; Lartigue, C.; Noskov, V. N.; Chuang, R.-Y.; Algire, M. A.; Benders, G. A.; Montague, M. G.; Ma, L.; Moodie, M. M.; Merryman, C.; Vashee, S.; Krishnakumar, R.; Assad-Garcia, N.; Andrews-Pfannkoch, C.; Denisova, E. A.; Young, L.; Qi, Z.-Q.; Segall-Shapiro, T. H.; Calvey, C. H.; Parmar, P. P.; Hutchison Ca, C. A.; Smith, H. O.; Venter, J. C. (2010). "Creation of a Bacterial Cell Controlled by a Chemically Synthesized Genome". Science. 329 (5987): 52–6. Bibcode:2010Sci...329...52G. doi:10.1126/science.1190719. PMID 20488990.
- ↑ Malyshev, Denis A.; Dhami, Kirandeep; Lavergne, Thomas; Chen, Tingjian; Dai, Nan; Foster, Jeremy M.; Corrêa, Ivan R.; Romesberg, Floyd E. (2014-05-15). "A semi-synthetic organism with an expanded genetic alphabet". Nature. 509 (7500): 385–388. Bibcode:2014Natur.509..385M. doi:10.1038/nature13314. PMC 4058825. PMID 24805238.
- ↑ Thyer, Ross; Ellefson, Jared (2014-05-15). "Synthetic biology: New letters for life's alphabet". Nature. 509 (7500): 291–292. Bibcode:2014Natur.509..291T. doi:10.1038/nature13335. PMID 24805244.
- ↑ Pollack, Andrew (2015-05-11). "Jennifer Doudna, a Pioneer Who Helped Simplify Genome Editing". The New York Times. Retrieved 2017-11-15.
- ↑ Jinek, Martin; Chylinski, Krzysztof; Fonfara, Ines; Hauer, Michael; Doudna, Jennifer A.; Charpentier, Emmanuelle (2012-08-17). "A Programmable Dual-RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity". Science. 337 (6096): 816–821. Bibcode:2012Sci...337..816J. doi:10.1126/science.1225829. PMID 22745249.
- ↑ Ledford, Heidi (2016-03-10). "CRISPR: gene editing is just the beginning". Nature. 531 (7593): 156–159. Bibcode:2016Natur.531..156L. doi:10.1038/531156a. PMID 26961639.
- ↑ Koh, Hee-Jong; Kwon, Suk-Yoon; Thomson, Michael (2015-08-26). Current Technologies in Plant Molecular Breeding: A Guide Book of Plant Molecular Breeding for Researchers. Springer. p. 242. ISBN 9789401799966.
- ↑ "How to Make a GMO - Science in the News". Science in the News. 2015-08-09. Retrieved 2017-04-29.
- ↑ Nicholl, Desmond S. T. (2008-05-29). An Introduction to Genetic Engineering. Cambridge University Press. p. 34. ISBN 9781139471787.
- ↑ Alberts B, Johnson A, Lewis J, et al. (2002). "8". Isolating, Cloning, and Sequencing DNA (4th ed.). New York: Garland Science.
- ↑ Kaufman, R I; Nixon, B T (1996). "Use of PCR to isolate genes encoding sigma54-dependent activators from diverse bacteria". J Bacteriol. 178 (13): 3967–3970. doi:10.1128/jb.178.13.3967-3970.1996. PMC 232662. PMID 8682806.
- ↑ Liang, Jing; Luo, Yunzi; Zhao, Huimin (2011). "Synthetic biology: Putting synthesis into biology". Wiley Interdisciplinary Reviews: Systems Biology and Medicine. 3: 7–20. doi:10.1002/wsbm.104. PMC 3057768. PMID 21064036.
- ↑ "5. THE PROCESS OF GENETIC MODIFICATION". www.fao.org. Retrieved 2017-04-29.
- ↑ Berg, P.; Mertz, J. E. (2010). "Personal Reflections on the Origins and Emergence of Recombinant DNA Technology". Genetics. 184 (1): 9–17. doi:10.1534/genetics.109.112144. PMC 2815933. PMID 20061565.
- ↑ Chen, I; Dubnau, D (2004). "DNA uptake during bacterial transformation". Nat. Rev. Microbiol. 2 (3): 241–9. doi:10.1038/nrmicro844. PMID 15083159.
- ↑ Health, National Research Council (US) Committee on Identifying and Assessing Unintended Effects of Genetically Engineered Foods on Human (2004-01-01). Methods and Mechanisms for Genetic Manipulation of Plants, Animals, and Microorganisms. National Academies Press (US).
- ↑ Gelvin, S. B. (2003). "Agrobacterium-Mediated Plant Transformation: The Biology behind the "Gene-Jockeying" Tool". Microbiology and Molecular Biology Reviews. 67 (1): 16–37, table of contents. doi:10.1128/MMBR.67.1.16-37.2003. PMC 150518. PMID 12626681.
- ↑ Head, Graham; Hull, Roger H; Tzotzos, George T. (2009). Genetically Modified Plants: Assessing Safety and Managing Risk. London: Academic Pr. p. 244. ISBN 0-12-374106-8.
- ↑ Darbani, Behrooz; Farajnia, Safar; Toorchi, Mahmoud; Zakerbostanabad, Saeed; Noeparvar, Shahin; Stewart, Jr., C. Neal (2010). "DNA-Delivery Methods to Produce Transgenic Plants". Science Alert.
- ↑ Tuomela, M.; Stanescu, I.; Krohn, K. (2005). "Validation overview of bio-analytical methods". Gene Therapy. 12 (S1): S131–S138. doi:10.1038/sj.gt.3302627. ISSN 0969-7128.
- ↑ Narayanaswamy, S. (1994). Plant Cell and Tissue Culture. Tata McGraw-Hill Education. pp. vi. ISBN 9780074602775.
- ↑ Health, National Research Council (US) Committee on Identifying and Assessing Unintended Effects of Genetically Engineered Foods on Human (2004). Methods and Mechanisms for Genetic Manipulation of Plants, Animals, and Microorganisms. National Academies Press (US).
- ↑ Hohn, Barbara; Levy, Avraham A; Puchta, Holger (2001). "Elimination of selection markers from transgenic plants". Current Opinion in Biotechnology. 12 (2): 139–43. doi:10.1016/S0958-1669(00)00188-9. PMID 11287227.
- ↑ Setlow, Jane K. (2002-10-31). Genetic Engineering: Principles and Methods. Springer Science & Business Media. p. 109. ISBN 9780306472800.
- ↑ Deepak, SA; Kottapalli, KR; Rakwal, R; Oros, G; Rangappa, KS; Iwahashi, H; Masuo, Y; Agrawal, GK (June 2007). "Real-Time PCR: Revolutionizing Detection and Expression Analysis of Genes". Current Genomics. 8 (4): 234–251. doi:10.2174/138920207781386960. ISSN 1389-2029. PMC 2430684. PMID 18645596.
- ↑ Grizot S, Smith J, Daboussi F, et al. (September 2009). "Efficient targeting of a SCID gene by an engineered single-chain homing endonuclease". Nucleic Acids Res. 37 (16): 5405–19. doi:10.1093/nar/gkp548. PMC 2760784. PMID 19584299.
- ↑ Gao H, Smith J, Yang M, et al. (January 2010). "Heritable targeted mutagenesis in maize using a designed endonuclease". Plant J. 61 (1): 176–87. doi:10.1111/j.1365-313X.2009.04041.x. PMID 19811621.
- ↑ Townsend JA, Wright DA, Winfrey RJ, et al. (May 2009). "High-frequency modification of plant genes using engineered zinc-finger nucleases". Nature. 459 (7245): 442–5. Bibcode:2009Natur.459..442T. doi:10.1038/nature07845. PMC 2743854. PMID 19404258.
- ↑ Shukla VK, Doyon Y, Miller JC, et al. (May 2009). "Precise genome modification in the crop species Zea mays using zinc-finger nucleases". Nature. 459 (7245): 437–41. Bibcode:2009Natur.459..437S. doi:10.1038/nature07992. PMID 19404259.
- ↑ Christian M, Cermak T, Doyle EL, et al. (July 2010). "TAL Effector Nucleases Create Targeted DNA Double-strand Breaks". Genetics. 186 (2): 757–61. doi:10.1534/genetics.110.120717. PMC 2942870. PMID 20660643.
- ↑ Li T, Huang S, Jiang WZ, et al. (August 2010). "TAL nucleases (TALNs): hybrid proteins composed of TAL effectors and FokI DNA-cleavage domain". Nucleic Acids Res. 39 (1): 359–72. doi:10.1093/nar/gkq704. PMC 3017587. PMID 20699274.
- ↑ Esvelt, KM.; Wang, HH. (2013). "Genome-scale engineering for systems and synthetic biology". Mol Syst Biol. 9: 641. doi:10.1038/msb.2012.66. PMC 3564264. PMID 23340847.
- ↑ Tan, WS.; Carlson, DF.; Walton, MW.; Fahrenkrug, SC.; Hackett, PB. (2012). "Precision editing of large animal genomes". Adv Genet. Advances in Genetics. 80: 37–97. doi:10.1016/B978-0-12-404742-6.00002-8. ISBN 9780124047426. PMC 3683964. PMID 23084873.
- 1 2 Malzahn, Aimee; Lowder, Levi; Qi, Yiping (2017-04-24). "Plant genome editing with TALEN and CRISPR". Cell & Bioscience. 7. doi:10.1186/s13578-017-0148-4. ISSN 2045-3701. PMC 5404292. PMID 28451378.
- ↑ Ekker, S.C. (2008). "Zinc finger-based knockout punches for zebrafish genes". Zebrafish. 5 (2): 1121–3. doi:10.1089/zeb.2008.9988. PMC 2849655. PMID 18554175.
- ↑ Geurts AM, Cost GJ, Freyvert Y, et al. (July 2009). "Knockout rats via embryo microinjection of zinc-finger nucleases". Science. 325 (5939): 433. Bibcode:2009Sci...325..433G. doi:10.1126/science.1172447. PMC 2831805. PMID 19628861.
- ↑ "Genetic Modification of Bacteria". Annenberg Foundation.
- ↑ Panesar, Pamit et al (2010) "Enzymes in Food Processing: Fundamentals and Potential Applications", Chapter 10, I K International Publishing House, ISBN 978-9380026336
- ↑ "GM traits list". International Service for the Acquisition of Agri-Biotech Applications.
- ↑ "ISAAA Brief 43-2011: Executive Summary". International Service for the Acquisition of Agri-Biotech Applications.
- ↑ Connor, Steve (2 November 2007). "The mouse that shook the world". The Independent.
- ↑ Avise, John C. (2004). The hope, hype & reality of genetic engineering: remarkable stories from agriculture, industry, medicine, and the environment. Oxford University Press US. p. 22. ISBN 978-0-19-516950-8.
- ↑ "Engineering algae to make complex anti-cancer 'designer' drug". PhysOrg. 10 December 2012. Retrieved 15 April 2013.
- ↑ =Roque, AC; Lowe, CR; Taipa, MA. (2004). "Antibodies and genetically engineered related molecules: production and purification". Biotechnol Proress. 20 (3): 639–54. doi:10.1021/bp030070k. PMID 15176864.
- ↑ Rodriguez, Luis L.; Grubman, Marvin J. (2009). "Foot and mouth disease virus vaccines". Vaccine. 27: D90–4. doi:10.1016/j.vaccine.2009.08.039. PMID 19837296.
- ↑ "Background: Cloned and Genetically Modified Animals". Center for Genetics and Society. 14 April 2005.
- ↑ "Knockout Mice". Nation Human Genome Research Institute. 2009.
- ↑ "GM pigs best bet for organ transplant". Medical News Today. 21 September 2003.
- ↑ Fischer, Alain; Hacein-Bey-Abina, Salima; Cavazzana-Calvo, Marina (2010). "20 years of gene therapy for SCID". Nature Immunology. 11 (6): 457–60. doi:10.1038/ni0610-457. PMID 20485269.
- ↑ Ledford, Heidi (2011). "Cell therapy fights leukaemia". Nature. doi:10.1038/news.2011.472.
- ↑ Brentjens, Renier J.; Davila, Marco L.; Riviere, Isabelle; Park, Jae; Wang, Xiuyan; Cowell, Lindsay G.; Bartido, Shirley; Stefanski, Jolanta; Taylor, Clare; Olszewska, Malgorzata; Borquez-Ojeda, Oriana; Qu, Jinrong; Wasielewska, Teresa; He, Qing; Bernal, Yvette; Rijo, Ivelise V.; Hedvat, Cyrus; Kobos, Rachel; Curran, Kevin; Steinherz, Peter; Jurcic, Joseph; Rosenblat, Todd; Maslak, Peter; Frattini, Mark; Sadelain, Michel (20 March 2013). "CD19-Targeted T Cells Rapidly Induce Molecular Remissions in Adults with Chemotherapy-Refractory Acute Lymphoblastic Leukemia". Science Translational Medicine. 5 (177): 177ra38–177ra38. doi:10.1126/scitranslmed.3005930. PMC 3742551. PMID 23515080.
- ↑ Lewitt, Peter A; Rezai, Ali R; Leehey, Maureen A; Ojemann, Steven G; Flaherty, Alice W; Eskandar, Emad N; Kostyk, Sandra K; Thomas, Karen; Sarkar, Atom; Siddiqui, Mustafa S; Tatter, Stephen B; Schwalb, Jason M; Poston, Kathleen L; Henderson, Jaimie M; Kurlan, Roger M; Richard, Irene H; Van Meter, Lori; Sapan, Christine V; During, Matthew J; Kaplitt, Michael G; Feigin, Andrew (2011). "AAV2-GAD gene therapy for advanced Parkinson's disease: A double-blind, sham-surgery controlled, randomised trial". The Lancet Neurology. 10 (4): 309–19. doi:10.1016/S1474-4422(11)70039-4. PMID 21419704.
- ↑ Gallagher, James. (2 November 2012) BBC News – Gene therapy: Glybera approved by European Commission. Bbc.co.uk. Retrieved on 15 December 2012.
- ↑ Richards, Sabrina. "Gene Therapy Arrives in Europe". The Scientist. Retrieved 16 November 2012.
- ↑ "Genetically Altered Skin Saves A Boy Dying Of A Rare Disease". NPR.org. Retrieved 2017-11-15.
- ↑ "1990 The Declaration of Inuyama". 5 August 2001. Archived from the original on 5 August 2001.
- ↑ Smith KR, Chan S, Harris J. Human germline genetic modification: scientific and bioethical perspectives. Arch Med Res. 2012 Oct;43(7):491-513. doi:10.1016/j.arcmed.2012.09.003. PMID 23072719
- ↑ Kolata, Gina (23 April 2015). "Chinese Scientists Edit Genes of Human Embryos, Raising Concerns". The New York Times. Retrieved 24 April 2015.
- ↑ Liang, Puping; et al. (18 April 2015). "CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes". Protein & Cell. 6: 363–72. doi:10.1007/s13238-015-0153-5. PMC 4417674. PMID 25894090. Retrieved 24 April 2015.
- ↑ Wade, Nicholas (3 December 2015). "Scientists Place Moratorium on Edits to Human Genome That Could Be Inherited". The New York Times. Retrieved 3 December 2015.
- ↑ Bergeson, Emilie R. (1997). "The Ethics of Gene Therapy".
- ↑ Hanna, Kathi E. "Genetic Enhancement". National Human Genome Research Institute.
- ↑ Harmon, Amy (2015-11-26). "Open Season Is Seen in Gene Editing of Animals". The New York Times. ISSN 0362-4331. Retrieved 2017-09-27.
- ↑ "Rediscovering Biology - Online Textbook: Unit 13 Genetically Modified Organisms". www.learner.org. Retrieved 2017-08-18.
- 1 2 3 Alberts, Bruce; Johnson, Alexander; Lewis, Julian; Raff, Martin; Roberts, Keith; Walter, Peter (2002). "Studying Gene Expression and Function".
- ↑ Park, Sheldon J.; Cochran, Jennifer R. (2009-09-25). Protein Engineering and Design. CRC Press. ISBN 9781420076592.
- ↑ Kurnaz, Isil Aksan (2015-05-08). Techniques in Genetic Engineering. CRC Press. ISBN 9781482260908.
- ↑ "Applications of Genetic Engineering". Microbiologyprocedure. Archived from the original on 14 July 2011. Retrieved 9 July 2010.
- ↑ "Biotech: What are transgenic organisms?". Easyscience. 2002. Archived from the original on 27 May 2010. Retrieved 9 July 2010.
- ↑ Savage, Neil (1 August 2007). "Making Gasoline from Bacteria: A biotech startup wants to coax fuels from engineered microbes". Technology Review. Retrieved 16 July 2015.
- ↑ Summers, Rebecca (24 April 2013) Bacteria churn out first ever petrol-like biofuel New Scientist, Retrieved 27 April 2013
- ↑ "Applications of Some Genetically Engineered Bacteria". Archived from the original on 27 November 2010. Retrieved 9 July 2010.
- ↑ Sanderson, Katherine (24 February 2012) New Portable Kit Detects Arsenic In Wells Chemical and Engineering News, Retrieved 23 January 2013
- ↑ Reece, Jane B.; Urry, Lisa A.; Cain, Michael L.; Wasserman, Steven A.; Minorsky, Peter V.; Jackson, Robert B. (2011). Campbell Biology Ninth Edition. San Francisco: Pearson Benjamin Cummings. p. 421. ISBN 0-321-55823-5.
- ↑ "New virus-built battery could power cars, electronic devices". Web.mit.edu. 2 April 2009. Retrieved 17 July 2010.
- ↑ "Hidden Ingredient In New, Greener Battery: A Virus". Npr.org. Retrieved 17 July 2010.
- ↑ "Researchers Synchronize Blinking 'Genetic Clocks' -- Genetically Engineered Bacteria That Keep Track of Time". ScienceDaily. 24 January 2010.
- ↑ Suszkiw, Jan (November 1999). "Tifton, Georgia: A Peanut Pest Showdown". Agricultural Research magazine. Retrieved 23 November 2008.
- ↑ Magaña-Gómez, JA; de la Barca, A.M. (2009). "Risk assessment of genetically modified crops for nutrition and health". Nutr. Rev. 67 (1): 1–16. doi:10.1111/j.1753-4887.2008.00130.x. PMID 19146501.
- ↑ Islam, Aparna (2008). "Fungus Resistant Transgenic Plants: Strategies, Progress and Lessons Learnt". Plant Tissue Culture and Biotechnology. 16 (2): 117–38. doi:10.3329/ptcb.v16i2.1113.
- ↑ "Disease resistant crops". GMO Compass. Archived from the original on 3 June 2010.
- ↑ Demont, M; Tollens, E (2004). "First impact of biotechnology in the EU: Bt maize adoption in Spain". Annals of Applied Biology. 145 (2): 197–207. doi:10.1111/j.1744-7348.2004.tb00376.x.
- ↑ Chivian, Eric; Bernstein, Aaron (2008). Sustaining Life. Oxford University Press, Inc. ISBN 978-0-19-517509-7.
- 1 2 Whitman, Deborah B. (2000). "Genetically Modified Foods: Harmful or Helpful?".
- ↑ Pollack, Andrew (19 November 2015). "Genetically Engineered Salmon Approved for Consumption". The New York Times. Retrieved 21 April 2016.
- ↑ Rapeseed (canola) has been genetically engineered to modify its oil content with a gene encoding a "12:0 thioesterase" (TE) enzyme from the California bay plant (Umbellularia californica) to increase medium length fatty acids, see: Geo-pie.cornell.edu Archived 5 July 2009 at the Wayback Machine.
- ↑ Bomgardner Melody M (2012). "Replacing Trans Fat: New crops from Dow Chemical and DuPont target food makers looking for stable, heart-healthy oils". Chemical and Engineering News. 90 (11): 30–32.
- ↑ Kramer, Matthew G.; Redenbaugh, Keith (1994-01-01). "Commercialization of a tomato with an antisense polygalacturonase gene: The FLAVR SAVR™ tomato story". Euphytica. 79 (3): 293–297. doi:10.1007/BF00022530. ISSN 0014-2336.
- ↑ Marvier, Michelle (2008). "Pharmaceutical crops in California, benefits and risks. A review". Agronomy for Sustainable Development. 28 (1): 1–9. doi:10.1051/agro:2007050.
- ↑ "FDA Approves First Human Biologic Produced by GE Animals". US Food and Drug Administration.
- ↑ Rebêlo, Paulo (15 July 2004). "GM cow milk 'could provide treatment for blood disease'". SciDev.
- ↑ Angulo, E.; Cooke, B. (2002). "First synthesize new viruses then regulate their release? The case of the wild rabbit". Molecular Ecology. 11: 2703–9. doi:10.1046/j.1365-294X.2002.01635.x. PMID 12453252.
- ↑ Adams; et al. (2 August 2002). "The Case for Genetic Engineering of Native and Landscape Trees against Introduced Pests and Diseases". Conservation Biology. 16: 874–879. doi:10.1046/j.1523-1739.2002.00523.x. Retrieved 16 May 2016.
- ↑ Thomas; et al. (25 September 2013). "Ecology: Gene tweaking for conservation". Nature. 501: 485–6. doi:10.1038/501485a. PMID 24073449. Retrieved 16 May 2016.
- ↑ Pasko, Jessica M. (2007-03-04). "Bio-artists bridge gap between arts, sciences: Use of living organisms is attracting attention and controversy". msnbc.
- ↑ Jackson, Joab (6 December 2005). "Genetically Modified Bacteria Produce Living Photographs". National Geographic News.
- ↑ Phys.Org website. 4 April 2005 "Plant gene replacement results in the world's only blue rose".
- ↑ Katsumoto, Yukihisa; Fukuchi-Mizutani, Masako; Fukui, Yuko; Brugliera, Filippa; Holton, Timothy A.; Karan, Mirko; Nakamura, Noriko; Yonekura-Sakakibara, Keiko; Togami, Junichi; Pigeaire, Alix; Tao, Guo-Qing; Nehra, Narender S.; Lu, Chin-Yi; Dyson, Barry K.; Tsuda, Shinzo; Ashikari, Toshihiko; Kusumi, Takaaki; Mason, John G.; Tanaka, Yoshikazu (2007). "Engineering of the Rose Flavonoid Biosynthetic Pathway Successfully Generated Blue-Hued Flowers Accumulating Delphinidin". Plant and Cell Physiology. 48 (11): 1589–600. doi:10.1093/pcp/pcm131. PMID 17925311.
- ↑ Published PCT Application WO2000049150 "Chimeric Gene Constructs for Generation of Fluorescent Transgenic Ornamental Fish." National University of Singapore
- ↑ Stewart, C. Neal (2006). "Go with the glow: Fluorescent proteins to light transgenic organisms" (PDF). Trends in Biotechnology. 24 (4): 155–62. doi:10.1016/j.tibtech.2006.02.002. PMID 16488034.
- ↑ Berg P, Baltimore D, Boyer HW, Cohen SN, Davis RW, Hogness DS, Nathans D, Roblin R, Watson JD, Weissman S, Zinder ND (1974). "Letter: Potential biohazards of recombinant DNA molecules" (PDF). Science. 185 (4148): 303. Bibcode:1974Sci...185..303B. doi:10.1126/science.185.4148.303. PMC 388511. PMID 4600381.
- ↑ Berg, P., Baltimore, D., Brenner, S., Roblin, R. O., and Singer, M. F. (1975). "Summary Statement of the Asilomar Conference on Recombinant DNA Molecules". Proc. Natl. Acad. Sci. USA. 72 (6): 1981–1984. Bibcode:1975PNAS...72.1981B. doi:10.1073/pnas.72.6.1981. PMC 432675. PMID 806076.
- ↑ McHughen A, Smyth S (2008). "US regulatory system for genetically modified [genetically modified organism (GMO), rDNA or transgenic] crop cultivars". Plant biotechnology journal. 6 (1): 2–12. doi:10.1111/j.1467-7652.2007.00300.x. PMID 17956539.
- 1 2 U.S. Office of Science and Technology Policy (1986). "Coordinated framework for regulation of biotechnology" (PDF). Fed. Regist. 51 (123): 23302–50. PMID 11655807. Archived from the original (PDF) on 16 May 2011.
- ↑ Redick, T.P. (2007). "The Cartagena Protocol on biosafety: Precautionary priority in biotech crop approvals and containment of commodities shipments, 2007". Colorado Journal of International Environmental Law and Policy. 18: 51–116.
- ↑ "About the Protocol". The Biosafety Clearing-House (BCH).
- ↑ "AgBioForum 13(3): Implications of Import Regulations and Information Requirements under the Cartagena Protocol on Biosafety for GM Commodities in Kenya".
- ↑ "Restrictions on Genetically Modified Organisms". Library of Congress. 9 June 2015. Retrieved 24 February 2016.
- ↑ Bashshur, Ramona (February 2013). "FDA and Regulation of GMOs". American Bar Association. Retrieved 24 February 2016.
- ↑ Sifferlin, Alexandra (3 October 2015). "Over Half of E.U. Countries Are Opting Out of GMOs". Time.
- ↑ Lynch, Diahanna; Vogel, David (5 April 2001). "The Regulation of GMOs in Europe and the United States: A Case-Study of Contemporary European Regulatory Politics". Council on Foreign Relations. Retrieved 24 February 2016.
- ↑ "Restrictions on Genetically Modified Organisms - Law Library of Congress". 22 January 2017.
- ↑ Emily Marden, Risk and Regulation: U.S. Regulatory Policy on Genetically Modified Food and Agriculture, 44 B.C.L. Rev. 733 (2003)
- 1 2 Davison, John (2010). "GM plants: Science, politics and EC regulations". Plant Science. 178 (2): 94–98. doi:10.1016/j.plantsci.2009.12.005.
- 1 2 GMO Compass: The European Regulatory System. Archived 14 August 2012 at the Wayback Machine. Retrieved 28 July 2012.
- ↑ Agency, Government of Canada, Canadian Food Inspection. "Information for the general public". www.inspection.gc.ca.
- ↑ Forsberg, Cecil W. (23 April 2013). "Genetically Modified Foods". The Canadian Encyclopedia. Retrieved 4 October 2017.
- ↑ Evans, Brent and Lupescu, Mihai (15 July 2012) Canada - Agricultural Biotechnology Annual – 2012 GAIN (Global Agricultural Information Network) report CA12029, United States Department of Agriculture, Foreifn Agricultural Service, Retrieved 5 November 2012
- ↑ McHugen, Alan (14 September 2000). "Chapter 1: Hors-d'oeuvres and entrees/What is genetic modification? What are GMOs?". Pandora's Picnic Basket. Oxford University Press. ISBN 978-0198506744.
- 1 2 Transgenic harvest Editorial, Nature 467, pages 633–634, 7 October 2010, doi:10.1038/467633b. Retrieved 9 November 2010
- ↑ "AgBioForum 5(4): Agricultural Biotechnology Development and Policy in China".
- ↑ "TNAU Agritech Portal :: Bio Technology".
- 1 2 3 "BASF presentation" (PDF). Archived from the original (PDF) on 28 September 2011.
- ↑ Agriculture - Department of Primary Industries Archived 29 March 2011 at the Wayback Machine.
- 1 2 "Welcome to the Office of the Gene Technology Regulator Website". Office of the Gene Technology Regulator. Retrieved 25 March 2011.
- ↑ "Regulation (EC) No 1829/2003 of the European Parliament and of the Council of 22 September 2003 On Genetically Modified Food And Feed" (PDF). Official Journal of the European Union. The European Parliament and the Council of the European Union. 2003. Archived from the original (PDF) on 20 January 2014.
The labeling should include objective information to the effect that a food or feed consists of, contains or is produced from GMOs. Clear labeling, irrespective of the detectability of DNA or protein resulting from the genetic modification in the final product, meets the demands expressed in numerous surveys by a large majority of consumers, facilitates informed choice and precludes potential misleading of consumers as regards methods of manufacture or production.
- ↑ "Regulation (EC) No 1830/2003 of the European Parliament and of the Council of 22 September 2003 concerning the traceability and labeling of genetically modified organisms and the traceability of food and feed products produced from genetically modified organisms and amending Directive 2001/18/EC". Official Journal L 268, 18/10/2003 P. 0024 - 0028. The European Parliament and the Council of the European Union. 2003.
(3) Traceability requirements for GMOs should facilitate both the withdrawal of products where unforeseen adverse effects on human health, animal health or the environment, including ecosystems, are established, and the targeting of monitoring to examine potential effects on, in particular, the environment. Traceability should also facilitate the implementation of risk management measures in accordance with the precautionary principle. (4) Traceability requirements for food and feed produced from GMOs should be established to facilitate accurate labeling of such products.
- ↑ "Report 2 of the Council on Science and Public Health: Labeling of Bioengineered Foods" (PDF). American Medical Association. 2012. Archived from the original (PDF) on 7 September 2012.
- ↑ American Association for the Advancement of Science (AAAS), Board of Directors (2012). Statement by the AAAS Board of Directors On Labeling of Genetically Modified Foods, and associated Press release: Legally Mandating GM Food Labels Could Mislead and Falsely Alarm Consumers
- ↑ Hallenbeck, Terri (2014-04-27). "How GMO labeling came to pass in Vermont". Burlington Free Press. Retrieved 2014-05-28.
- ↑ "The Regulation of Genetically Modified Foods".
- ↑ Sheldon, Ian M. (2002-03-01). "Regulation of biotechnology: will we ever 'freely' trade GMOs?". European Review of Agricultural Economics. 29 (1): 155–176. CiteSeerX 10.1.1.596.7670. doi:10.1093/erae/29.1.155. ISSN 0165-1587.
- ↑ Dabrock, Peter (2017-05-05). "Playing God? Synthetic biology as a theological and ethical challenge". Systems and Synthetic Biology. 3 (1–4): 47–54. doi:10.1007/s11693-009-9028-5. ISSN 1872-5325. PMC 2759421. PMID 19816799.
- ↑ Brown, Carolyn (2000-10-03). "Patenting life: genetically altered mice an invention, court declares". CMAJ: Canadian Medical Association Journal. 163 (7): 867–868. ISSN 0820-3946. PMC 80518. PMID 11033718.
- ↑ Zhou, Wen (2015-08-10). "The Patent Landscape of Genetically Modified Organisms - Science in the News". Science in the News. Retrieved 2017-05-05.
- ↑ Puckett, Lily (2016-04-20). "Why The New GMO Food-Labeling Law Is So Controversial". Huffington Post. Retrieved 2017-05-05.
- ↑ Miller, Henry (2016-04-12). "GMO food labels are meaningless". Los Angeles Times. ISSN 0458-3035. Retrieved 2017-05-05.
- ↑ Savage, Steven. "Who Controls The Food Supply?". Forbes. Retrieved 2017-05-05.
- ↑ Knight, Andrew J. (2016-04-14). Science, Risk, and Policy. Routledge. p. 156. ISBN 9781317280811.
- ↑ Hakim, Danny (2016-10-29). "Doubts About the Promised Bounty of Genetically Modified Crops". The New York Times. ISSN 0362-4331. Retrieved 2017-05-05.
- ↑ Areal, F. J.; Riesgo, L.; Rodríguez-Cerezo, E. (2013-02-01). "Economic and agronomic impact of commercialized GM crops: a meta-analysis". The Journal of Agricultural Science. 151 (1): 7–33. doi:10.1017/S0021859612000111. ISSN 0021-8596.
- ↑ Finger, Robert; El Benni, Nadja; Kaphengst, Timo; Evans, Clive; Herbert, Sophie; Lehmann, Bernard; Morse, Stephen; Stupak, Nataliya (2011-05-10). "A Meta Analysis on Farm-Level Costs and Benefits of GM Crops". Sustainability. 3 (5): 743–762. doi:10.3390/su3050743.
- ↑ Klümper, Wilhelm; Qaim, Matin (2014-11-03). "A Meta-Analysis of the Impacts of Genetically Modified Crops". PLOS ONE. 9 (11): e111629. Bibcode:2014PLoSO...9k1629K. doi:10.1371/journal.pone.0111629. ISSN 1932-6203. PMC 4218791. PMID 25365303.
- ↑ Qiu, Jane (2013). "Genetically modified crops pass benefits to weeds". Nature. doi:10.1038/nature.2013.13517.
- 1 2 "GMOs and the environment". www.fao.org. Retrieved 2017-05-07.
- ↑ Dively, Galen P.; Venugopal, P. Dilip; Finkenbinder, Chad (2016-12-30). "Field-Evolved Resistance in Corn Earworm to Cry Proteins Expressed by Transgenic Sweet Corn". PLOS ONE. 11 (12): e0169115. Bibcode:2016PLoSO..1169115D. doi:10.1371/journal.pone.0169115. ISSN 1932-6203. PMC 5201267. PMID 28036388.
- ↑ Qiu, Jane (2010-05-13). "GM crop use makes minor pests major problem". Nature News. doi:10.1038/news.2010.242.
- ↑ Gilbert, Natasha (2013-05-02). "Case studies: A hard look at GM crops". Nature. 497 (7447): 24–26. Bibcode:2013Natur.497...24G. doi:10.1038/497024a. PMID 23636378.
- ↑ "Are GMO Fish Safe for the Environment? | Accumulating Glitches | Learn Science at Scitable". www.nature.com. Retrieved 2017-05-07.
- ↑ "Q&A: genetically modified food". World Health Organization. Retrieved 2017-05-07.
- ↑ Nicolia, Alessandro; Manzo, Alberto; Veronesi, Fabio; Rosellini, Daniele (2013). "An overview of the last 10 years of genetically engineered crop safety research" (PDF). Critical Reviews in Biotechnology. 34: 1–12. doi:10.3109/07388551.2013.823595. PMID 24041244.
We have reviewed the scientific literature on GE crop safety for the last 10 years that catches the scientific consensus matured since GE plants became widely cultivated worldwide, and we can conclude that the scientific research conducted so far has not detected any significant hazard directly connected with the use of GM crops.
The literature about Biodiversity and the GE food/feed consumption has sometimes resulted in animated debate regarding the suitability of the experimental designs, the choice of the statistical methods or the public accessibility of data. Such debate, even if positive and part of the natural process of review by the scientific community, has frequently been distorted by the media and often used politically and inappropriately in anti-GE crops campaigns.
- ↑ "State of Food and Agriculture 2003–2004. Agricultural Biotechnology: Meeting the Needs of the Poor. Health and environmental impacts of transgenic crops". Food and Agriculture Organization of the United Nations. Retrieved 8 February 2016.
Currently available transgenic crops and foods derived from them have been judged safe to eat and the methods used to test their safety have been deemed appropriate. These conclusions represent the consensus of the scientific evidence surveyed by the ICSU (2003) and they are consistent with the views of the World Health Organization (WHO, 2002). These foods have been assessed for increased risks to human health by several national regulatory authorities (inter alia, Argentina, Brazil, Canada, China, the United Kingdom and the United States) using their national food safety procedures (ICSU). To date no verifiable untoward toxic or nutritionally deleterious effects resulting from the consumption of foods derived from genetically modified crops have been discovered anywhere in the world (GM Science Review Panel). Many millions of people have consumed foods derived from GM plants - mainly maize, soybean and oilseed rape - without any observed adverse effects (ICSU).
- ↑ Ronald, Pamela (5 May 2011). "Plant Genetics, Sustainable Agriculture and Global Food Security". Genetics. 188: 11–20. doi:10.1534/genetics.111.128553. PMC 3120150. PMID 21546547.
There is broad scientific consensus that genetically engineered crops currently on the market are safe to eat. After 14 years of cultivation and a cumulative total of 2 billion acres planted, no adverse health or environmental effects have resulted from commercialization of genetically engineered crops (Board on Agriculture and Natural Resources, Committee on Environmental Impacts Associated with Commercialization of Transgenic Plants, National Research Council and Division on Earth and Life Studies 2002). Both the U.S. National Research Council and the Joint Research Centre (the European Union's scientific and technical research laboratory and an integral part of the European Commission) have concluded that there is a comprehensive body of knowledge that adequately addresses the food safety issue of genetically engineered crops (Committee on Identifying and Assessing Unintended Effects of Genetically Engineered Foods on Human Health and National Research Council 2004; European Commission Joint Research Centre 2008). These and other recent reports conclude that the processes of genetic engineering and conventional breeding are no different in terms of unintended consequences to human health and the environment (European Commission Directorate-General for Research and Innovation 2010).
- ↑ But see also:
Domingo, José L.; Bordonaba, Jordi Giné (2011). "A literature review on the safety assessment of genetically modified plants" (PDF). Environment International. 37: 734–742. doi:10.1016/j.envint.2011.01.003. PMID 21296423.
In spite of this, the number of studies specifically focused on safety assessment of GM plants is still limited. However, it is important to remark that for the first time, a certain equilibrium in the number of research groups suggesting, on the basis of their studies, that a number of varieties of GM products (mainly maize and soybeans) are as safe and nutritious as the respective conventional non-GM plant, and those raising still serious concerns, was observed. Moreover, it is worth mentioning that most of the studies demonstrating that GM foods are as nutritional and safe as those obtained by conventional breeding, have been performed by biotechnology companies or associates, which are also responsible of commercializing these GM plants. Anyhow, this represents a notable advance in comparison with the lack of studies published in recent years in scientific journals by those companies.
Krimsky, Sheldon (2015). "An Illusory Consensus behind GMO Health Assessment" (PDF). Science, Technology, & Human Values. 40: 1–32. doi:10.1177/0162243915598381.I began this article with the testimonials from respected scientists that there is literally no scientific controversy over the health effects of GMOs. My investigation into the scientific literature tells another story.
And contrast:Panchin, Alexander Y.; Tuzhikov, Alexander I. (14 January 2016). "Published GMO studies find no evidence of harm when corrected for multiple comparisons". Critical Reviews in Biotechnology: 1–5. doi:10.3109/07388551.2015.1130684. ISSN 0738-8551. PMID 26767435.
Here, we show that a number of articles some of which have strongly and negatively influenced the public opinion on GM crops and even provoked political actions, such as GMO embargo, share common flaws in the statistical evaluation of the data. Having accounted for these flaws, we conclude that the data presented in these articles does not provide any substantial evidence of GMO harm.
The presented articles suggesting possible harm of GMOs received high public attention. However, despite their claims, they actually weaken the evidence for the harm and lack of substantial equivalency of studied GMOs. We emphasize that with over 1783 published articles on GMOs over the last 10 years it is expected that some of them should have reported undesired differences between GMOs and conventional crops even if no such differences exist in reality. and
Yang, Y.T.; Chen, B. (2016). "Governing GMOs in the USA: science, law and public health". Journal of the Science of Food and Agriculture. 96: 1851–1855. doi:10.1002/jsfa.7523. PMID 26536836.
It is therefore not surprising that efforts to require labeling and to ban GMOs have been a growing political issue in the USA (citing Domingo and Bordonaba, 2011).
Overall, a broad scientific consensus holds that currently marketed GM food poses no greater risk than conventional food... Major national and international science and medical associations have stated that no adverse human health effects related to GMO food have been reported or substantiated in peer-reviewed literature to date.
Despite various concerns, today, the American Association for the Advancement of Science, the World Health Organization, and many independent international science organizations agree that GMOs are just as safe as other foods. Compared with conventional breeding techniques, genetic engineering is far more precise and, in most cases, less likely to create an unexpected outcome.
- ↑ "Statement by the AAAS Board of Directors On Labeling of Genetically Modified Foods" (PDF). American Association for the Advancement of Science. 20 October 2012. Retrieved 8 February 2016.
The EU, for example, has invested more than €300 million in research on the biosafety of GMOs. Its recent report states: "The main conclusion to be drawn from the efforts of more than 130 research projects, covering a period of more than 25 years of research and involving more than 500 independent research groups, is that biotechnology, and in particular GMOs, are not per se more risky than e.g. conventional plant breeding technologies." The World Health Organization, the American Medical Association, the U.S. National Academy of Sciences, the British Royal Society, and every other respected organization that has examined the evidence has come to the same conclusion: consuming foods containing ingredients derived from GM crops is no riskier than consuming the same foods containing ingredients from crop plants modified by conventional plant improvement techniques.
Pinholster, Ginger (25 October 2012). "AAAS Board of Directors: Legally Mandating GM Food Labels Could "Mislead and Falsely Alarm Consumers"". American Association for the Advancement of Science. Retrieved 8 February 2016. - ↑ "A decade of EU-funded GMO research (2001–2010)" (PDF). Directorate-General for Research and Innovation. Biotechnologies, Agriculture, Food. European Commission, European Union. 2010. doi:10.2777/97784. ISBN 978-92-79-16344-9. Retrieved 8 February 2016.
- ↑ "AMA Report on Genetically Modified Crops and Foods (online summary)". American Medical Association. January 2001. Retrieved 19 March 2016.
A report issued by the scientific council of the American Medical Association (AMA) says that no long-term health effects have been detected from the use of transgenic crops and genetically modified foods, and that these foods are substantially equivalent to their conventional counterparts. (from online summary prepared by ISAAA)" "Crops and foods produced using recombinant DNA techniques have been available for fewer than 10 years and no long-term effects have been detected to date. These foods are substantially equivalent to their conventional counterparts. (from original report by AMA: )
"REPORT 2 OF THE COUNCIL ON SCIENCE AND PUBLIC HEALTH (A-12): Labeling of Bioengineered Foods" (PDF). American Medical Association. 2012. Archived from the original on 7 September 2012. Retrieved 19 March 2016.Bioengineered foods have been consumed for close to 20 years, and during that time, no overt consequences on human health have been reported and/or substantiated in the peer-reviewed literature.
- ↑ "Restrictions on Genetically Modified Organisms: United States. Public and Scholarly Opinion". Library of Congress. 9 June 2015. Retrieved 8 February 2016.
Several scientific organizations in the US have issued studies or statements regarding the safety of GMOs indicating that there is no evidence that GMOs present unique safety risks compared to conventionally bred products. These include the National Research Council, the American Association for the Advancement of Science, and the American Medical Association. Groups in the US opposed to GMOs include some environmental organizations, organic farming organizations, and consumer organizations. A substantial number of legal academics have criticized the US's approach to regulating GMOs.
- ↑ "Genetically Engineered Crops: Experiences and Prospects". The National Academies of Sciences, Engineering, and Medicine (US). 2016. p. 149. Retrieved 19 May 2016.
Overall finding on purported adverse effects on human health of foods derived from GE crops: On the basis of detailed examination of comparisons of currently commercialized GE with non-GE foods in compositional analysis, acute and chronic animal toxicity tests, long-term data on health of livestock fed GE foods, and human epidemiological data, the committee found no differences that implicate a higher risk to human health from GE foods than from their non-GE counterparts.
- ↑ "Frequently asked questions on genetically modified foods". World Health Organization. Retrieved 8 February 2016.
Different GM organisms include different genes inserted in different ways. This means that individual GM foods and their safety should be assessed on a case-by-case basis and that it is not possible to make general statements on the safety of all GM foods.
GM foods currently available on the international market have passed safety assessments and are not likely to present risks for human health. In addition, no effects on human health have been shown as a result of the consumption of such foods by the general population in the countries where they have been approved. Continuous application of safety assessments based on the Codex Alimentarius principles and, where appropriate, adequate post market monitoring, should form the basis for ensuring the safety of GM foods.
- ↑ Haslberger, Alexander G. (2003). "Codex guidelines for GM foods include the analysis of unintended effects". Nature Biotechnology. 21: 739–741. doi:10.1038/nbt0703-739. PMID 12833088.
These principles dictate a case-by-case premarket assessment that includes an evaluation of both direct and unintended effects.
- ↑ Some medical organizations, including the British Medical Association, advocate further caution based upon the precautionary principle:
"Genetically modified foods and health: a second interim statement" (PDF). British Medical Association. March 2004. Retrieved 21 March 2016.
In our view, the potential for GM foods to cause harmful health effects is very small and many of the concerns expressed apply with equal vigour to conventionally derived foods. However, safety concerns cannot, as yet, be dismissed completely on the basis of information currently available.
When seeking to optimise the balance between benefits and risks, it is prudent to err on the side of caution and, above all, learn from accumulating knowledge and experience. Any new technology such as genetic modification must be examined for possible benefits and risks to human health and the environment. As with all novel foods, safety assessments in relation to GM foods must be made on a case-by-case basis.
Members of the GM jury project were briefed on various aspects of genetic modification by a diverse group of acknowledged experts in the relevant subjects. The GM jury reached the conclusion that the sale of GM foods currently available should be halted and the moratorium on commercial growth of GM crops should be continued. These conclusions were based on the precautionary principle and lack of evidence of any benefit. The Jury expressed concern over the impact of GM crops on farming, the environment, food safety and other potential health effects.
The Royal Society review (2002) concluded that the risks to human health associated with the use of specific viral DNA sequences in GM plants are negligible, and while calling for caution in the introduction of potential allergens into food crops, stressed the absence of evidence that commercially available GM foods cause clinical allergic manifestations. The BMA shares the view that that there is no robust evidence to prove that GM foods are unsafe but we endorse the call for further research and surveillance to provide convincing evidence of safety and benefit.
- ↑ Funk, Cary; Rainie, Lee (29 January 2015). "Public and Scientists' Views on Science and Society". Pew Research Center. Retrieved 24 February 2016.
The largest differences between the public and the AAAS scientists are found in beliefs about the safety of eating genetically modified (GM) foods. Nearly nine-in-ten (88%) scientists say it is generally safe to eat GM foods compared with 37% of the general public, a difference of 51 percentage points.
- ↑ Marris, Claire (2001). "Public views on GMOs: deconstructing the myths". EMBO Reports. 2: 545–548. doi:10.1093/embo-reports/kve142. PMC 1083956. PMID 11463731.
- ↑ Final Report of the PABE research project (December 2001). "Public Perceptions of Agricultural Biotechnologies in Europe". Commission of European Communities. Retrieved 24 February 2016.
- ↑ Scott, Sydney E.; Inbar, Yoel; Rozin, Paul (2016). "Evidence for Absolute Moral Opposition to Genetically Modified Food in the United States" (PDF). Perspectives on Psychological Science. 11 (3): 315–324. doi:10.1177/1745691615621275. PMID 27217243.
Further reading
- British Medical Association (1999). The Impact of Genetic Modification on Agriculture, Food and Health. BMJ Books. ISBN 0-7279-1431-6.
- Donnellan, Craig (2004). Genetic Modification (Issues). Independence Educational Publishers. ISBN 1-86168-288-3.
- Morgan, Sally (1 January 2009). Superfoods: Genetic Modification of Foods. Heinemann Library. ISBN 978-1-4329-2455-3.
- Smiley, Sophie (2005). Genetic Modification: Study Guide (Exploring the Issues). Independence Educational Publishers. ISBN 1-86168-307-3.
- Watson, James D. (2007). Recombinant DNA: Genes and Genomes: A Short Course. San Francisco: W.H. Freeman. ISBN 0-7167-2866-4.
- Weaver, Sean; Michael, Morris (2003). "An Annotated Bibliography of Scientific Publications on the Risks Associated with Genetic Modification". Wellington, N.Z.: Victoria University
- Zaid, A; Hughes, H.G.; Porceddu, E.; Nicholas, F. (2001). Glossary of Biotechnology for Food and Agriculture - A Revised and Augmented Edition of the Glossary of Biotechnology and Genetic Engineering. Rome, Italy: FAO. ISBN 92-5-104683-2.
External links
| Library resources about Genetic engineering |
| Wikimedia Commons has media related to Genetic engineering. |
| At Wikiversity, you can learn more and teach others about Genetic engineering at the Department of Genetic engineering |