Arabidopsis thaliana
| Arabidopsis thaliana | |
|---|---|
 | |
| Scientific classification | |
| Kingdom: | Plantae |
| Clade: | Angiosperms |
| Clade: | Eudicots |
| Clade: | Rosids |
| Order: | Brassicales |
| Family: | Brassicaceae |
| Genus: | Arabidopsis |
| Species: | A. thaliana |
| Binomial name | |
| Arabidopsis thaliana (L.) Heynh. | |
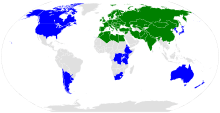 | |
The range of Arabidopsis thaliana.
| |
| Synonyms[1] | |
|
Arabis thaliana | |
Arabidopsis thaliana, the thale cress, mouse-ear cress or arabidopsis, is a small flowering plant native to Eurasia and Africa.[2][3][4][5][6][7] A. thaliana is considered a weed; it is found by roadsides and in disturbed land.
A winter annual with a relatively short life cycle, A. thaliana is a popular model organism in plant biology and genetics. For a complex multicellular eukaryote, A. thaliana has a relatively small genome of approximately 135 megabase pairs (Mbp).[8] It was the first plant to have its genome sequenced, and is a popular tool for understanding the molecular biology of many plant traits, including flower development and light sensing.
Description
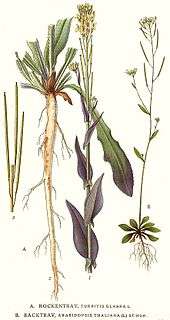
Arabidopsis thaliana is an annual (rarely biennial) plant, usually growing to 20–25 cm tall.[6] The leaves form a rosette at the base of the plant, with a few leaves also on the flowering stem. The basal leaves are green to slightly purplish in color, 1.5–5 cm long and 2–10 mm broad, with an entire to coarsely serrated margin; the stem leaves are smaller and unstalked, usually with an entire margin. Leaves are covered with small, unicellular hairs (called trichomes). The flowers are 3 mm in diameter, arranged in a corymb; their structure is that of the typical Brassicaceae. The fruit is a siliqua 5–20 mm long, containing 20–30 seeds.[9][10][11][12] Roots are simple in structure, with a single primary root that grows vertically downward, later producing smaller lateral roots. These roots form interactions with rhizosphere bacteria such as Bacillus megaterium.[13]
A. thaliana can complete its entire lifecycle in six weeks. The central stem that produces flowers grows after about three weeks, and the flowers naturally self-pollinate. In the lab, A. thaliana may be grown in Petri plates, pots, or hydroponics, under fluorescent lights or in a greenhouse.[14]
Taxonomy
The plant was first described in 1577 in the Harz Mountains by Johannes Thal (1542–1583), a physician from Nordhausen, Thüringen, Germany, who called it Pilosella siliquosa. In 1753, Carl Linnaeus renamed the plant Arabis thaliana in honor of Thal. In 1842, the German botanist Gustav Heynhold erected the new genus Arabidopsis and placed the plant in that genus. The genus name, Arabidopsis, comes from Greek, meaning "resembling Arabis" (the genus in which Linnaeus had initially placed it).
Thousands of natural inbred accessions of A. thaliana have been collected from throughout its natural and introduced range.[15] These accessions exhibit considerable genetic and phenotypic variation which can be used to study the adaptation of this species to different environments.[15]
Distribution and habitat
A. thaliana is native to Europe, Asia, Africa. It also appears to be native in tropical afroalpine ecosystems.[16] It has been introduced and naturalized worldwide.[17]
A. thaliana readily grows and often pioneers rocky, sandy and calcareous soils. It is generally considered a weed, due to its widespread distribution in agricultural fields, roadside, railway lines, waste ground and other disturbed habitat.[17][18]
Like most Brassicaceae species, A. thaliana is edible by humans as a salad or cooked, but it does not enjoy a widespread use as a spring vegetable.[17]
Use as a model organism
Botanists and biologists began to research A. thaliana in the early 1900s, and the first systematic description of mutants was done around 1945.[19] A. thaliana is now widely used for studying plant sciences, including genetics, evolution, population genetics, and plant development.[20][21][22] Although A. thaliana has little direct significance for agriculture, it has several traits that make it a useful model for understanding the genetic, cellular, and molecular biology of flowering plants.
The first mutant in A. thaliana was documented in 1873 by Alexander Braun, describing a double flower phenotype (the mutated gene was likely Agamous, cloned and characterized in 1990).[23] However, not until 1943 did Friedrich Laibach (who had published the chromosome number in 1907) propose A. thaliana as a model organism.[24] His student, Erna Reinholz, published her thesis on A. thaliana in 1945, describing the first collection of A. thaliana mutants that they generated using X-ray mutagenesis. Laibach continued his important contributions to A. thaliana research by collecting a large number of accessions (often questionably referred to as 'ecotypes'). With the help of Albert Kranz, these were organised into a large collection of 750 natural accessions of A. thaliana from around the world.

In the 1950s and 1960s, John Langridge and George Rédei played an important role in establishing A. thaliana as a useful organism for biological laboratory experiments. Rédei wrote several scholarly reviews instrumental in introducing the model to the scientific community. The start of the A. thaliana research community dates to a newsletter called Arabidopsis Information Service (AIS), established in 1964. The first International Arabidopsis Conference was held in 1965, in Göttingen, Germany.
In the 1980s, A. thaliana started to become widely used in plant research laboratories around the world. It was one of several candidates that included maize, petunia, and tobacco.[24] The latter two were attractive, since they were easily transformable with the then-current technologies, while maize was a well-established genetic model for plant biology. 1986 was a breakthrough year for A. thaliana as a model plant, in which T-DNA-mediated transformation and the first cloned A. thaliana gene were described.[25][26]
Genome
The small size of its genome, and the fact that it is diploid, makes Arabidopsis thaliana useful for genetic mapping and sequencing — with about 135 mega base pairs[27] and five chromosomes, A. thaliana has one of the smallest genomes among plants.[8] It was long thought to have the smallest genome of all flowering plants,[28] but that title is now considered to belong to plants in the genus Genlisea, order Lamiales, with Genlisea tuberosa, a carnivorous plant, showing a genome size of approximately 61 Mbp.[29] It was the first plant genome to be sequenced, completed in 2000 by the Arabidopsis Genome Initiative.[30] The most up-to-date version of the A. thaliana genome is maintained by the Arabidopsis Information Resource (TAIR).[31] Much work has been done to assign functions to its 27,000 genes and the 35,000 proteins they encode.[32] Post-genomic research, such as metabolomics, has also provided useful insights to the metabolism of this species and how environmental perturbations [33] can affect metabolic processes.[34]
Trichome formation is initiated by the GLABROUS1 protein. Knockouts of the corresponding gene lead to glabrous plants. This phenotype has already been used in gene editing experiments and might be of interest as visual marker for plant research to improve gene editing methods such as CRISPR/Cas9.[35][36]
Genetics
Genetic transformation of A. thaliana is routine, utilizing Agrobacterium tumefaciens to transfer DNA into the plant genome. The current protocol, termed "floral dip", involves simply dipping flowers into a solution containing Agrobacterium carrying a plasmid of interest and a detergent.[37][38] This method avoids the need for tissue culture or plant regeneration.
The A. thaliana gene knockout collections are a unique resource for plant biology made possible by the availability of high-throughput transformation and funding for genomics resources. The site of T-DNA insertions has been determined for over 300,000 independent transgenic lines, with the information and seeds accessible through online T-DNA databases. Through these collections, insertional mutants are available for most genes in A. thaliana.
Characterized accessions and mutant lines of A. thaliana serve as experimental material in laboratory studies. The most commonly used background lines are Ler (Landsberg erecta), and Col, or Columbia.[39] Other background lines less-often cited in the scientific literature are Ws, or Wassilewskija, C24, Cvi, or Cape Verde Islands, Nossen, etc. (see for ex.[40]) Sets of closely related accessions named Col-0, Col-1, etc., have been obtained and characterized; in general, mutant lines are available through stock centers, of which best-known are the Nottingham Arabidopsis Stock Center-NASC[39] and the Arabidopsis Biological Resource Center-ABRC in Ohio, USA.[41] The Col-0 accession was selected by Rédei from within a (nonirradiated) population of seeds designated 'Landsberg' which he received from Laibach.[42] Columbia (named for the location of Rédei's former institution, the University of Missouri-Columbia) was the reference accession sequenced in the Arabidopsis Genome Initiative. The Ler (Landsberg erecta) line was selected by Rédei (because of its short stature) from a Landsberg population he had mutagenized with X-rays. As the Ler collection of mutants is derived from this initial line, Ler-0 does not correspond to the Landsberg accessions, which designated La-0, La-1, etc.
Non-Mendelian inheritance controversy
In 2005, scientists at Purdue University proposed that A. thaliana possessed an alternative to previously known mechanisms of DNA repair, producing an unusual pattern of inheritance. However, the phenomenon observed (reversion of mutant copies of the HOTHEAD gene to a wild-type state) was later suggested to be an artifact because the mutants show increased outcrossing due to organ fusion.[43][44][45]
Life cycle
The plant's small size and rapid lifecycle are also advantageous for research. Having specialized as a spring ephemeral, it has been used to found several laboratory strains that take about six weeks from germination to mature seed. The small size of the plant is convenient for cultivation in a small space, and it produces many seeds. Further, the selfing nature of this plant assists genetic experiments. Also, as an individual plant can produce several thousand seeds; each of the above criteria leads to A. thaliana being valued as a genetic model organism.
Development
Flower development
A. thaliana has been extensively studied as a model for flower development. The developing flower has four basic organs: sepals, petals, stamens, and carpels (which go on to form pistils). These organs are arranged in a series of whorls: four sepals on the outer whorl, followed by four petals inside this, six stamens, and a central carpel region. Homeotic mutations in A. thaliana result in the change of one organ to another — in the case of the agamous mutation, for example, stamens become petals and carpels are replaced with a new flower, resulting in a recursively repeated sepal-petal-petal pattern.
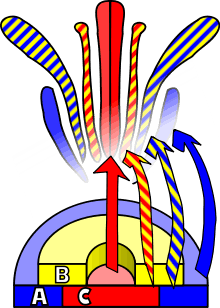
Observations of homeotic mutations led to the formulation of the ABC model of flower development by E. Coen and E. Meyerowitz.[46] According to this model, floral organ identity genes are divided into three classes: class A genes (which affect sepals and petals), class B genes (which affect petals and stamens), and class C genes (which affect stamens and carpels). These genes code for transcription factors that combine to cause tissue specification in their respective regions during development. Although developed through study of A. thaliana flowers, this model is generally applicable to other flowering plants.
Leaf development
Studies of A. thaliana have provided considerable insights with regards to the genetics of leaf morphogenesis, particularly in dicotyledon-type plants.[47][48] Much of the understanding has come from analyzing mutants in leaf development, some of which were identified in the 1960s, but were not analysed with genetic and molecular techniques until the mid-1990s. A. thaliana leaves are well suited to studies of leaf development because they are relatively simple and stable.
Using A. thaliana, the genetics behind leaf shape development have become more clear and have been broken down into three stages: The initiation of the leaf primordium, the establishment of dorsiventrality, and the development of a marginal meristem. Leaf primordia are initiated by the suppression of the genes and proteins of the class I KNOX family (such as SHOOT APICAL MERISTEMLESS). These class I KNOX proteins directly suppress gibberellin biosynthesis in the leaf primordium. Many genetic factors were found to be involved in the suppression of these class I KNOX genes in leaf primordia (such as ASYMMETRIC LEAVES1, BLADE-ON-PETIOLE1, SAWTOOTH1, etc.). Thus, with this suppression, the levels of gibberellin increase and leaf primordium initiates growth.
The establishment of leaf dorsiventrality is important since the dorsal (adaxial) surface of the leaf is different from the ventral (abaxial) surface.[49]
Microscopy
A. thaliana is well suited for light microscopy analysis. Young seedlings on the whole, and their roots in particular, are relatively translucent. This, together with their small size, facilitates live cell imaging using both fluorescence and confocal laser scanning microscopy.[50] By wet-mounting seedlings in water or in culture media, plants may be imaged uninvasively, obviating the need for fixation and sectioning and allowing time-lapse measurements.[51] Fluorescent protein constructs can be introduced through transformation. The developmental stage of each cell can be inferred from its location in the plant or by using fluorescent protein markers, allowing detailed developmental analysis.
Physiology
Light sensing, light emission, and circadian biology
The photoreceptors phytochromes A, B, C, D, and E mediate red light-based phototropic response. Understanding the function of these receptors has helped plant biologists understand the signalling cascades that regulate photoperiodism, germination, de-etiolation, and shade avoidance in plants.
The UVR8 protein detects UV-B light and mediates response to this DNA damaging wavelength.
A. thaliana was used extensively in the study of the genetic basis of phototropism, chloroplast alignment, and stomatal aperture and other blue light-influenced processes.[52] These traits respond to blue light, which is perceived by the phototropin light receptors. Arabidopsis has also been important in understanding the functions of another blue light receptor, cryptochrome, which is especially important for light entrainment to control the plants' circadian rhythms.[53] When the onset of darkness is unusually early, A. thaliana reduces its metabolism of starch by an amount that effectively requires division.[54]
Light responses were even found in roots, previously thought to be largely insensitive to light. While the gravitropic response of A. thaliana root organs is their predominant tropic response, specimens treated with mutagens and selected for the absence of gravitropic action showed negative phototropic response to blue or white light, and positive response to red light, indicating that the roots also show positive phototropism.[55]
In 2000, Dr. Janet Braam of Rice University genetically engineered A. thaliana to glow in the dark when touched. The effect was visible to ultrasensitive cameras.[56]
Multiple efforts, including the Glowing Plant project, have sought to use A. thaliana to increase plant luminescence intensity towards commercially viable levels.
Plant–pathogen interactions
It is important to understand how plants achieve resistance to protect the world's food production, as well as the agriculture industry. Many model systems have been developed to better understand interactions between plants and bacterial, fungal, oomycete, viral, and nematode pathogens. Arabidopsis thaliana has been a powerful tool for the study of the subdicipline of plant pathology, that is, the interaction between plants and disease-causing pathogens.
| Pathogen type | Example in Arabidopsis thaliana |
|---|---|
| Bacteria | Pseudomonas syringae, Xanthomonas campestris |
| Fungi | Colletotrichum destructivum, Botrytis cinerea, Golovinomyces orontii |
| Oomycete | Hyaloperonospora arabidopsidis |
| Viral | Cauliflower mosaic virus (CaMV), tomato mosaic virus (TMV) |
| Nematode | Meloidogyne incognita, Heterodera schachtii |
The use of A. thaliana has led to many breakthroughs in the advancement of knowledge of how plants manifest plant disease resistance. The reason most plants are resistant to most pathogens is through nonhost resistance. This is, not all pathogens will infect all plants. An example where A. thaliana was used to determine the genes responsible for nonhost resistance is Blumeria graminis, the causal agent of powdery mildew of grasses. A. thaliana mutants were developed using the mutagen ethyl methanesulfonate and screened to identify mutants with increased infection by B. graminis.[57][58][59] The mutants with higher infection rates are referred to as PEN mutants due to the ability of B. graminis to penetrate A. thaliana to begin the disease process. The PEN genes were later mapped to identify the genes responsible for nonhost resistance to B. graminis.
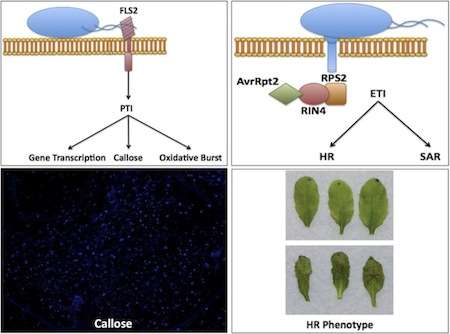
A schematic of PAPM-triggered immunity, to be specific recognition of flagellin by FLS2 (top left), effector-triggered immunity depicted through the recognition of avrRpt2 by RPS2 through RIN4 (top-right), microscopic view of callose deposition in an A. thaliana leaf (bottom left), an example of no hypersensitive response (HR), top, and HR in A. thaliana leaves (bottom right)
In general, when a plant is exposed to a pathogen, or nonpathogenic microbe, there is an initial response, known as PAMP-triggered immunity (PTI), because the plant detects conserved motifs known as pathogen-associated molecular patterns (PAMPs).[60] These PAMPs are detected by specialized receptors in the host known as pattern recognition receptors (PRRs) on the plant cell surface.
The best-characterized PRR in A. thaliana is FLS2 (Flagellin-Sensing2), which recognizes bacterial flagellin,[61][62] a specialized organelle used by microorganisms for the purpose of motility, as well as the ligand flg22, which comprises the 22 amino acids recognized by FLS2. Discovery of FLS2 was facilitated by the identification of an A. thaliana ecotype, Ws-0, that was unable to detect flg22, leading to the identification of the gene encoding FLS2. FLS2 shows striking similarity to rice XA21, the first PRR isolated in 1995
A second PRR, EF-Tu receptor (EFR), identified in A. thaliana, recognizes the bacterial EF-Tu protein, the prokaryotic elongation factor used in protein synthesis, as well as the laboratory-used ligand elf18.[63] Using Agrobacterium-mediated transformation, a technique that takes advantage of the natural process by which Agrobacterium transfers genes into host plants, the EFR gene was transformed into Nicotiana benthamiana, tobacco plant that does not recognize EF-Tu, thereby permitting recognition of bacterial EF-Tu[64] thereby confirming EFR as the receptor of EF-Tu.
Both FLS2 and EFR use similar signal transduction pathways to initiate PTI. A. thaliana has been instrumental in dissecting these pathways to better understand the regulation of immune responses, the most notable one being the mitogen-activated protein kinase (MAP kinase) cascade. Downstream responses of PTI include callose deposition, the oxidative burst, and transcription of defense-related genes.[65]
PTI is able to combat pathogens in a nonspecific manner. A stronger and more specific response in plants is that of effector-triggered immunity (ETI). ETI is dependent upon the recognition of pathogen effectors, proteins secreted by the pathogen that alter functions in the host, by plant resistance genes (R-genes), often described as a gene-for-gene relationship. This recognition may occur directly or indirectly via a guardee protein in a hypothesis known as the guard hypothesis. The first R-gene cloned in A. thaliana was RPS2 (resistance to Pseudomonas syringe 2), which is responsible for recognition of the effector avrRpt2.[66] The bacterial effector avrRpt2 is delivered into A. thaliana via the Type III secretion system of P. syringae pv tomato strain DC3000. Recognition of avrRpt2 by RPS2 occurs via the guardee protein RIN4, which is cleaved . Recognition of a pathogen effector leads to a dramatic immune response known as the hypersensitive response, in which the infected plant cells undergo cell death to prevent the spread of the pathogen.[67]
Systemic acquired resistance (SAR) is another example of resistance that is better understood in plants because of research done in A. thaliana. Benzothiadiazol (BTH), a salicylic acid (SA) analog, has been used historically as an antifungal compound in crop plants. BTH, as well as SA, has been shown to induce SAR in plants. The initiation of the SAR pathway was first demonstrated in A. thaliana in which increased SA levels are recognized by nonexpresser of PR genes 1 (NPR1)[68] due to redox change in the cytosol, resulting in the reduction of NPR1. NPR1, which usually exists in a multiplex (oligomeric) state, becomes monomeric (a single unit) upon reduction.[69] When NPR1 becomes monomeric, it translocates to the nucleus, were it interacts with many TGA transcription factors, and is able to induce pathogen-related genes such as PR1.[70] Another example of SAR would be the research done with transgenic tobacco plants, which express bacterial salicylate hydroxylase, nahG gene, requires the accumulation of SA for its expression [71]
Evolutionary aspect of plant-pathogen resistance
Plants are affected by multiple pathogens throughout their lifetime. In response to the presence of pathogens, plants have evolved receptors on the cell surface to detect and respond to pathogens.[72] Arabidopsis Thaliana is a model organism used to determine specific defense mechanisms of plant-pathogen resistance.[73] These plants have special receptors on their cell surfaces that allow for detection of pathogens and initiate mechanisms to inhibit pathogen growth.[73] They contain two receptors, FLS2 (bacterial flagellin receptor) and EF-Tu (bacterial EF-Tu protein), which use signal transduction pathways to initiate the disease response pathway.[73] The pathway leads to the recognition of the pathogen causing the infected cells to undergo cell death to stop the spread of the pathogen.[73] Plants with FLS2 and EF-Tu receptors have shown to have increased fitness in the population.[71] This has led to the belief that plant-pathogen resistance is an evolutionary mechanism that has built up over generations to respond to dynamic environments, such as increased predation and extreme temperatures.[71]
A. thaliana has also been used to study systemic acquired resistance (SAR).[74] This pathway utilizes Benzothiadiazol, a chemical inducer, to induce transcription factors, mRNA, of SAR genes. This accumulation of transcription factors leads to inhibition of pathogen-related genes.[74]
Plant-pathogen interactions are important for understanding of how plants have evolved to combat different types of pathogens that may affect them.[71] Variation in resistance of plants across populations is due to variation in environmental factors. Plants that have evolved resistance, whether it be the general variation or the SAR variation, have been able to live longer and hold off necrosis of their tissue (premature death of cells), which leads to better adaptation and fitness for populations that are in rapidly changing environments.[71]
Other research
Ongoing research on Arabidopsis thaliana is being performed on the International Space Station by the European Space Agency. The goals are to study the growth and reproduction of plants from seed to seed in microgravity.[75][76]
'Plant on a chip' devices in which A. thaliana tissues can be cultured in semi in-vitro conditions have been described.[77] Use of these devices may aid our understanding of pollen tube guidance and the mechanism of sexual reproduction in A. thaliana.
Self-pollination
A. thaliana is a predominantly self-pollinating plant with an outcrossing rate estimated at less than 0.3%.[78] An analysis of the genome-wide pattern of linkage disequilibrium suggested that self-pollination evolved roughly a million years ago or more.[79] Meioses that lead to self-pollination are unlikely to produce significant beneficial genetic variability. However, these meioses can provide the adaptive benefit of recombinational repair of DNA damages during formation of germ cells at each generation.[80] Such a benefit may have been sufficient to allow the long-term persistence of meioses even when followed by self-fertilization. A physical mechanism for self-pollination in A. thaliana is through pre-anthesis autogamy, such that fertilisation takes place largely before flower opening.
Databases and other resources
- TAIR and NASC: curated sources for diverse genetic and molecular biology information, links to gene expression databases etc.
- Arabidopsis Biological Resource Center (Seed and DNA stocks)
- Nottingham Arabidopsis Stock Centre (Seed and DNA stocks)
See also
References
- ↑ Warwick, S.I.; Francis, A.; Al-Shehbaz, I.A. (2016). "Brassicaceae species checklist and database". Species 2000 & ITIS Catalogue of Life (26 ed.). ISSN 2405-8858.
- ↑ "Arabidopsis thaliana". Germplasm Resources Information Network (GRIN). Agricultural Research Service (ARS), United States Department of Agriculture (USDA). Retrieved 11 December 2017.
- ↑ Hoffmann, Matthias H. (2002). "Biogeography of Arabidopsis thaliana (L.) Heynh. (Brassicaceae)". Journal of Biogeography. 29: 125–134. doi:10.1046/j.1365-2699.2002.00647.x.
- ↑ Mitchell-Olds, Thomas (December 2001). "Arabidopsis thaliana and its wild relatives: a model system for ecology and evolution". Trends in Ecology & Evolution. 16 (12): 693–700. doi:10.1016/s0169-5347(01)02291-1.
- ↑ Sharbel, Timothy F.; Haubold, Bernhard; Mitchell-Olds, Thomas (2000). "Genetic isolation by distance in Arabidopsis thaliana: biogeography and postglacial colonization of Europe". Molecular Ecology. 9 (12): 2109–2118. doi:10.1046/j.1365-294x.2000.01122.x.
- 1 2 Krämer, Ute (2015-03-25). "Planting molecular functions in an ecological context with Arabidopsis thaliana". eLife. 4: –06100. doi:10.7554/eLife.06100. ISSN 2050-084X. PMC 4373673. PMID 25807084. Retrieved 2017-04-20.
- ↑ Durvasula A, Fulgione A, Gutaker RM, Alacakaptan SI, Flood PJ, Neto C, Tsuchimatsu T, Burbano HA, Xavier Picó F, Alonso-Blanco C, Hancock AM (May 2017). "African genomes illuminate the early history and transition to selfing in Arabidopsis thaliana". Proceedings of the National Academy of Sciences. 114 (20): 5213–5218. doi:10.1073/pnas.1616736114. PMC 5441814. PMID 28473417.
- 1 2 "Genome Assembly". The Arabidopsis Information Resource. Retrieved 29 March 2016.
- ↑ Flora of NW Europe: Arabidopsis thaliana Archived 8 December 2007 at the Wayback Machine.
- ↑ Blamey, M. & Grey-Wilson, C. (1989). Flora of Britain and Northern Europe. ISBN 0-340-40170-2
- ↑ Flora of Pakistan: Arabidopsis thaliana
- ↑ Flora of China: Arabidopsis thaliana
- ↑ López-Bucio J, Campos-Cuevas JC, Hernández-Calderón E, et al. (2007). "Bacillus megaterium rhizobacteria promote growth and alter root-system architecture through an auxin- and ethylene-independent signaling mechanism in Arabidopsis thaliana". Mol. Plant Microbe Interact. 20 (2): 207–17. doi:10.1094/MPMI-20-2-0207. PMID 17313171.
- ↑ D.W. Meinke; J.M. Cherry; C. Dean; S.D. Rounsley; M. Koornneef (1998). "Arabidopsis thaliana: A Model Plant for Genome Analysis". Science. 282 (5389): 662–682. Bibcode:1998Sci...282..662M. CiteSeerX 10.1.1.462.4735. doi:10.1126/science.282.5389.662. PMID 9784120.
- 1 2 1001 Genomes Consortium (2016). "1,135 Genomes Reveal the Global Pattern of Polymorphism in "Arabidopsis thaliana"". Cell. 166 (2): 481–491. doi:10.1016/j.cell.2016.05.063. PMC 4949382. PMID 27293186.
- ↑ Hedberg, Olov (1957). "Afroalpine Vascular Plants: A Taxonomic Revision". Acta Universitatis Upsaliensis: Symbolae Botanicae Upsalienses. 15 (1): 1–144.
- 1 2 3 "Arabidopsis thaliana – Overview". Encyclopedia of Life.
- ↑ "Arabidopsis thaliana (thale cress)". Kew Gardens.
- ↑ TAIR: About Arabidopsis
- ↑ Rensink WA, Buell CR (2004). "Arabidopsis to Rice. Applying Knowledge from a Weed to Enhance Our Understanding of a Crop Species". Plant Physiol. 135 (2): 622–9. doi:10.1104/pp.104.040170. PMC 514098. PMID 15208410.
- ↑ Coelho SM, Peters AF, Charrier B, et al. (2007). "Complex life cycles of multicellular eukaryotes: new approaches based on the use of model organisms". Gene. 406 (1–2): 152–70. doi:10.1016/j.gene.2007.07.025. PMID 17870254.
- ↑ Platt A, Horton M, Huang YS, Li Y, Anastasio AE, et al. (2010). Novembre J, ed. "The Scale of Population Structure in Arabidopsis thaliana". PLOS Genetics. 6 (2): e1000843. doi:10.1371/journal.pgen.1000843. PMC 2820523. PMID 20169178.
- ↑ M.F. Yanofsky; H. Ma; J.L. Bowman; G.N. Drews; K.A. Feldmann; E.M. Meyerowitz (1990). "The protein encoded by the Arabidopsis homeotic gene agamous resembles transcription factors". Nature. 346 (6279): 35–39. Bibcode:1990Natur.346...35Y. doi:10.1038/346035a0. PMID 1973265.
- 1 2 E.M. Meyerowitz (2001). "Prehistory and History of Arabidopsis Research". Plant Physiology. 125 (1): 15–19. doi:10.1104/pp.125.1.15. PMC 1539315. PMID 11154286.
- ↑ Lloyd AM, Barnason AR, Rogers SG, Byrne MC, Fraley RT, Horsch RB (1986). "Transformation of Arabidopsis thaliana with Agrobacterium tumefaciens". Science. 234 (4775): 464–466. Bibcode:1986Sci...234..464L. doi:10.1126/science.234.4775.464. PMID 17792019.
- ↑ Chang C, Meyerowitz EM (1986). "Molecular cloning and DNA sequence of the Arabidopsis thaliana alcohol dehydrogenase gene". Proc Natl Acad Sci USA. 83 (5): 1408–1412. Bibcode:1986PNAS...83.1408C. doi:10.1073/pnas.83.5.1408. PMC 323085. PMID 2937058.
- ↑ Bennett, M. D., Leitch, I. J., Price, H. J., & Johnston, J. S. (2003). "Comparisons with Caenorhabditis (100 Mb) and Drosophila (175 Mb) Using Flow Cytometry Show Genome Size in Arabidopsis to be 157 Mb and thus 25% Larger than the Arabidopsis Genome Initiative Estimate of 125 Mb". Annals of Botany. 91 (5): 547–557. doi:10.1093/aob/mcg057. PMC 4242247. PMID 12646499.
- ↑ (Leutwileret al., 1984). In our survey Arabidopsis ...
- ↑ Fleischmann A, Michael TP, Rivadavia F, Sousa A, Wang W, Temsch EM, Greilhuber J, Müller KF, Heubl G (2014). "Evolution of genome size and chromosome number in the carnivorous plant genus Genlisea (Lentibulariaceae), with a new estimate of the minimum genome size in angiosperms". Annals of Botany. 114 (8): 1651–1663. doi:10.1093/aob/mcu189. PMC 4649684. PMID 25274549.
- ↑ The Arabidopsis Genome Initiative (2000). "Analysis of the genome sequence of the flowering plant Arabidopsis thaliana". Nature. 408 (6814): 796–815. Bibcode:2000Natur.408..796T. doi:10.1038/35048692. PMID 11130711.
- ↑ "TAIR - Genome Annotation".
- ↑ "Integr8 - A.thaliana Genome Statistics".
- ↑ Bundy, JG; Davey, MP; Viant, MR (2009). "Environmental Metabolomics: A Critical Review and Future Perspectives. (Invited Review)". Metabolomics. 5 (3–21).
- ↑ Lake, JA; Field, KJ; Davey, MP; Beerling, DJ,; Lomax, BH (2009). "Metabolomic and physiological responses reveal multi-phasic acclimation of Arabidopsis thaliana to chronic UV radiation". Plant Cell and Environment (32): 1377–1389.
- ↑ Hahn, Florian; Mantegazza, Otho; Greiner, André; Hegemann, Peter; Eisenhut, Marion; Weber, Andreas P. M. (2017). "An Efficient Visual Screen for CRISPR/Cas9 Activity in Arabidopsis thaliana". Frontiers in Plant Science. 8: 39. doi:10.3389/fpls.2017.00039. ISSN 1664-462X. PMC 5258748. PMID 28174584.
- ↑ Hahn, Florian; Eisenhut, Marion; Mantegazza, Otho; Weber, Andreas P. M. (5 April 2018). "Homology-Directed Repair of a Defective Glabrous Gene in Arabidopsis With Cas9-Based Gene Targeting". Frontiers in Plant Science. 9: 424. doi:10.3389/fpls.2018.00424. PMC 5895730. PMID 29675030.
- ↑ Clough SJ, Bent AF (1998). "Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana". Plant J. 16 (6): 735–743. doi:10.1046/j.1365-313x.1998.00343.x. PMID 10069079.
- ↑ Zhang X, Henriques R, Lin SS, Niu QW, Chua NH (2006). "Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method". Nat Protoc. 1 (2): 641–6. doi:10.1038/nprot.2006.97. PMID 17406292.
- 1 2 NASC-Nottingham Arabidopsis Stock Center - http://arabidopsis.info
- ↑ Alconada Magliano, Teresa M.; Botto, Javier F.; Godoy, A. Veronica; Symonds, V. Vaughan; Lloyd, Alan M.; Casal, Jorge J. (2005). "New Arabidopsis Recombinant Inbred Lines (Landsberg erecta x Nossen) Reveal Natural Variation in Phytochrome-Mediated Responses". Plant Physiology. 138 (2): 1126–1135. doi:10.1104/pp.104.059071. PMC 1150426. PMID 15908601.
- ↑ The Arabidopsis Biological Resource Center (ABRC), http://abrc.osu.edu
- ↑ NASC-Nottingham Arabidopsis Stock Center-Background Lines-Description- http://arabidopsis.info/CollectionInfo?id=94
- ↑ Lolle SJ, Victor JL, Young JM, Pruitt RE (2005). "Genome-wide non-mendelian inheritance of extra-genomic information in Arabidopsis". Nature. 434 (7032): 505–9. Bibcode:2005Natur.434..505L. doi:10.1038/nature03380. PMID 15785770. Washington Post summary.
- ↑ Peng P.; et al. (2006). "Plant genetics: Increased outcrossing in hothead mutants". Nature. 443 (7110): E8–E9. Bibcode:2006Natur.443E...8P. doi:10.1038/nature05251. PMID 17006468.
- ↑ Pennisi E (2006). "Genetics. Pollen contamination may explain controversial inheritance". Science. 313 (5795): 1864. doi:10.1126/science.313.5795.1864. PMID 17008492.
- ↑ Enrico S. Coen; Elliot M. Meyerowitz (1991). "The war of the whorls: Genetic interactions controlling flower development". Nature. 353 (6339): 31–37. Bibcode:1991Natur.353...31C. doi:10.1038/353031a0. PMID 1715520.
- ↑ Tsukaya, Hirokazu (2013-06-07). "Leaf Development". The Arabidopsis Book / American Society of Plant Biologists. 11: e0163. doi:10.1199/tab.0163. ISSN 1543-8120. PMC 3711357. PMID 23864837.
- ↑ Turner, Simon; Sieburth, Leslie E. (2003-03-22). "Vascular Patterning". The Arabidopsis Book / American Society of Plant Biologists. 2: e0073. doi:10.1199/tab.0073. ISSN 1543-8120. PMC 3243335. PMID 22303224.
- ↑ Efroni, Idan; Eshed, Yuval; Lifschitz, Eliezer (2010-04-01). "Morphogenesis of Simple and Compound Leaves: A Critical Review". The Plant Cell. 22 (4): 1019–1032. doi:10.1105/tpc.109.073601. ISSN 1040-4651. PMC 2879760. PMID 20435903.
- ↑ Moreno N, Bougourd S, Haseloff J and Fiejo JA. 2006. Chapter 44: Imaging Plant Cells. In: Pawley JB (Editor). Handbook of Biological Confocal Microscopy - 3rd edition. SpringerScience+Business Media, New York. p769-787
- ↑ Shaw S (2006). "Imaging the live plant cell". The Plant Journal. 45 (4): 573–598. doi:10.1111/j.1365-313X.2006.02653.x. PMID 16441350.
- ↑ Sullivan JA, Deng XW (2003). "From seed to seed: the role of photoreceptors in Arabidopsis development". Dev. Biol. 260 (2): 289–97. doi:10.1016/S0012-1606(03)00212-4. PMID 12921732.
- ↑ Más P (2005). "Circadian clock signaling in Arabidopsis thaliana: from gene expression to physiology and development". Int. J. Dev. Biol. 49 (5–6): 491–500. doi:10.1387/ijdb.041968pm. PMID 16096959.
- ↑ Scialdone, Antonio; Mugford, Sam T; Feike, Doreen; Skeffington, Alastair; Borrill, Philippa; Graf, Alexander; Smith, Alison M; Howard, Martin (25 June 2013). "Arabidopsis plants perform arithmetic division to prevent starvation at night". eLife. 2: e00669. doi:10.7554/eLife.00669. PMC 3691572. PMID 23805380.
- ↑ Ruppel NJ, Hangarter RP, Kiss JZ (2001). "Red-light-induced positive phototropism in arabidopsis roots". Planta. 212 (3): 424–30. doi:10.1007/s004250000410. PMID 11289607.
- ↑ "Plants that Glow in the Dark", Bioresearch Online, 18 May 2000
- ↑ Collins, NC; Thordal-Christensen H; Lipka V; Bau S; Kombrink E; Qiu JL; Hückelhoven R; Stein M; Freialdenhoven A; Somerville SC; Schulze-Lefert P (30 Oct 2003). "SNARE-protein-mediated disease resistance at the plant cell wall". Nature. 425 (6961): 973–7. Bibcode:2003Natur.425..973C. doi:10.1038/nature02076. PMID 14586469.
- ↑ Lipka V, Dittgen J, Bednarek P, Bhat R, Wiermer M, Stein M, Landtag J, Brandt W, Rosahl S, Scheel D, Llorente F, Molina A, Parker J, Somerville S, Schulze-Lefert P (18 November 2005). "Pre- and postinvasion defenses both contribute to nonhost resistance in Arabidopsis" (PDF). Science. 310 (5751): 1180–3. Bibcode:2005Sci...310.1180L. doi:10.1126/science.1119409. hdl:11858/00-001M-0000-0012-3A32-0. PMID 16293760.
- ↑ Stein, M; Dittgen J; Sánchez-Rodríguez C; Hou BH; Molina A; Schulze-Lefert P; Lipka V; Somerville S. (18 March 2006). "Arabidopsis PEN3/PDR8, an ATP Binding Cassette Transporter, Contributes to Nonhost Resistance to Inappropriate Pathogens That Enter by Direct Penetration". Plant Cell. 18 (3): 731–46. doi:10.1105/tpc.105.038372. PMC 1383646. PMID 16473969.
- ↑ Knepper, Caleb; Day, Brad (March 2010). "From Perception to Activation: The Molecular-Genetic and Biochemical Landscape of Disease Resistance Signaling in Plants". The Arabidopsis Book. 8: 1–17. doi:10.1199/tab.0124. PMC 3244959. PMID 22303251.
- ↑ Gomez-Gomez, L; Felix G; Boller T (18 May 1999). "A single locus determines sensitivity to bacterial flagellin in Arabidopsis thaliana". Plant J. 18 (3): 277–84. doi:10.1046/j.1365-313X.1999.00451.x. PMID 10377993.
- ↑ Gomez-Gomez, L; Boller T (5 June 2000). "FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis". Mol Cell. 5 (6): 1003–11. doi:10.1016/S1097-2765(00)80265-8. PMID 10911994.
- ↑ Zipfel, C; Kunze G; Chinchilla D; Caniard A; Jones JD; Boller T; Felix G (19 May 2006). "Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation". Cell. 125 (4): 749–60. doi:10.1016/j.cell.2006.03.037. PMID 16713565.
- ↑ Lacombe S, Rougon-Cardoso A, Sherwood E, Peeters N, Dahlbeck D, van Esse HP, Smoker M, Rallapalli G, Thomma BP, Staskawicz B, Jones JD, Zipfel C (28 April 2010). "Interfamily transfer of a plant pattern-recognition receptor confers broad-spectrum bacterial resistance". Nat Biotechnol. 28 (4): 365–9. doi:10.1038/nbt.1613. PMID 20231819. ,
- ↑ Zhang, J; Zhou JM (3 September 2010). "Plant immunity triggered by microbial molecular signatures". Mol Plant. 3 (5): 783–93. doi:10.1093/mp/ssq035. PMID 20713980.
- ↑ Kunkel, BN; Bent AF; Dahlbeck D; Innes RW; Staskawicz BJ. (5 August 1993). "RPS2, an Arabidopsis disease resistance locus specifying recognition of Pseudomonas syringae strains expressing the avirulence gene avrRpt2". Plant Cell. 5 (8): 865–75. doi:10.1105/tpc.5.8.865. PMC 160322. PMID 8400869.
- ↑ Axtell, MJ,; Staskawicz BJ (7 February 2003). "Initiation of RPS2-specified disease resistance in Arabidopsis is coupled to the AvrRpt2-directed elimination of RIN4". Cell. 112 (3): 369–77. doi:10.1016/S0092-8674(03)00036-9. PMID 12581526.
- ↑ Cao, H; Bowling SA; Gordon AS; Dong X. (6 November 1994). "Characterization of an Arabidopsis Mutant That Is Nonresponsive to Inducers of Systemic Acquired Resistance". Plant Cell. 6 (11): 1583–1592. doi:10.1105/tpc.6.11.1583. PMC 160545. PMID 12244227.
- ↑ Mou, Z; Fan W; Dong X. (27 June 2003). "Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes". Cell. 113 (7): 935–44. doi:10.1016/S0092-8674(03)00429-X. PMID 12837250.
- ↑ Johnson, C; Boden E; Arias J. (15 August 2003). "Salicylic Acid and NPR1 Induce the Recruitment of trans-Activating TGA Factors to a Defense Gene Promoter in Arabidopsis". Plant Cell. 15 (8): 1846–58. doi:10.1105/tpc.012211. PMC 167174. PMID 12897257.
- 1 2 3 4 5 Delaney, T.; Uknes, S.; Vernooij, B. (1994). "A Central Role of Salicylic Acid in Plant Disease Resistance". Science. 266 (5188): 1247–1252. Bibcode:1994Sci...266.1247D. doi:10.1126/science.266.5188.1247. PMID 17810266.
- ↑ Bent, AF.; Kunkel, BN.; Dahlbeck, D. (1994). "RPS2 of Arabidopsis thaliana: a leucine-rich repeat class of plant disease resistance genes". Science. 265 (5180): 1856–1860. Bibcode:1994Sci...265.1856B. doi:10.1126/science.8091210.
- 1 2 3 4 Zipfel, C.; Robatzek, S.; Navarro, L. (2004). "Bacterial disease resistance in Arabidopsis through flagellin perception". Nature. 428 (6984): 764–767. Bibcode:2004Natur.428..764Z. doi:10.1038/nature02485. PMID 15085136.
- 1 2 Lawton, K.; Friedrich, L.; Hunt, M. (1996). "Benzothiadizaole induces disease resistance by a citation of the systemic acquired resistance signal transduction pathway". The Plant Journal. 10: 71–82. doi:10.1046/j.1365-313x.1996.10010071.x.
- ↑ Link, Bruce M., Busse, James S., Stankovic, Bratislav (2014) Seed-to-Seed-to-Seed Growth and Development of Arabidopsis in Microgravity. Astrobiology Vol. 14: Issue. 10: Pages. 866-875 doi:10.1089/ast.2014.1184
- ↑ Ferl RJ1, Paul AL (Apr 2010). "Lunar plant biology--a review of the Apollo era". Astrobiology. 10 (3): 261–74. Bibcode:2010AsBio..10..261F. doi:10.1089/ast.2009.0417. PMID 20446867.
- ↑ AK Yetisen; L Jiang; J R Cooper; Y Qin; R Palanivelu; Y Zohar (May 2011). "A microsystem-based assay for studying pollen tube guidance in plant reproduction". J. Micromech. Microeng. 25.
- ↑ Abbott, RJ; Gomes, MF (1989). "Population genetic structure and outcrossing rate of Arabidopsis thaliana (L.) Heynh". Heredity. 62 (3): 411–418. doi:10.1038/hdy.1989.56.
- ↑ Tang C, Toomajian C, Sherman-Broyles S, Plagnol V, Guo YL, Hu TT, Clark RM, Nasrallah JB, Weigel D, Nordborg M (August 2007). "The evolution of selfing in Arabidopsis thaliana". Science. 317 (5841): 1070–2. Bibcode:2007Sci...317.1070T. doi:10.1126/science.1143153. PMID 17656687.
- ↑ Harris Bernstein, Carol Bernstein and Richard E. Michod (2011). Meiosis as an Evolutionary Adaptation for DNA Repair. Chapter 19 in DNA Repair. Inna Kruman editor. InTech Open Publisher. doi:10.5772/25117 http://www.intechopen.com/books/dna-repair/meiosis-as-an-evolutionary-adaptation-for-dna-repair
External links
| Wikimedia Commons has media related to Arabidopsis thaliana. |
- Arabidopsis transcriptional regulatory map
- The Arabidopsis Information Resource (TAIR)
- Salk Institute Genomic Analysis Laboratory
- What Makes Plants Grow? The Arabidopsis genome knows Featured article in Genome News Network
- The Arabidopsis book - A comprehensive review published yearly related to research in Arabidopsis
- A. thaliana protein abundance
- The Arabidopsis Information Portal (Araport)