Extraction (chemistry)
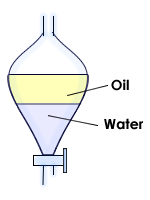

Extraction in chemistry is a separation process consisting in the separation of a substance from a matrix. It includes Liquid-liquid extraction, and Solid phase extraction. The distribution of a solute between two phases is an equilibrium condition described by partition theory. This is based on exactly how the analyte move from the water into an organic layer.
Types of extraction
There exist several types of extraction, including: liquid–liquid extraction, solid-phase extraction,and acid-base extraction. In liquid-liquid extraction compounds separate according to their relative solubility in two different immiscible liquid phases. This technique has been applied in several fields such as analytical chemistry and biology.[1]
Components of an extractive process
Extractions often use two immiscible phases to separate a solute from one phase into the other. Typical lab extractions are of organic compounds out of an aqueous phase and into an organic phase. Common extractants are arranged from ethyl acetate to water (ethyl acetate < acetone < ethanol < methanol < acetone:water (7:3) < ethanol:water (8:2) < methanol:water (8:2) < water) in increasing order of polarity according to the Hildebrand solubility parameter. The extract can be put back to dried form using a centrifugal evaporator or a freeze-drier.
Modern variations and Applications
Techniques include supercritical carbon dioxide extraction, ultrasonic extraction, heat reflux extraction, mechanochemical-assisted extraction, microwave-assisted extraction, instant controlled pressure drop extraction (DIC), and perstraction.
Boiling tea leaves in water extracts the tannins, theobromine, and caffeine out of the leaves and into the water. Solid-liquid extractions at laboratory scales can use Soxhlet extractors (such as oil from olive cake see at right).
Examples
Popular home examples of extractants include:
Further reading
- Gunt Hamburg, 2014, Thermal Process Engineering: Liquid-liquid extraction and solid-liquid extraction, see , accessed 12 May 2014.
- G.W. Stevens, T.C., Lo, & M. H. I. Baird, 2007, "Extraction, Liquid-Liquid", in Kirk-Othmer Encyclopedia of Chemical Technology, DOI: 10.1002/0471238961.120917211215.a01.pub2, see , accessed 12 May 2014.
- T. Voeste, K. Weber, B. Hiskey & G. Brunner, 2006, "Liquid–Solid Extraction", in Ullmann's Encyclopedia of Industrial Chemistry, DOI: 10.1002/14356007.b03_07.pub2, see , accessed 12 May 2014.
- R. J. Wakeman, 2000, "Extraction, Liquid-Solid", in Kirk-Othmer Encyclopedia of Chemical Technology, DOI: 10.1002/0471238961.1209172123011105.a01, see , accessed 12 May 2014.
- M.J.M. Wells, 2000, "Essential guides to method development in solid-phase extraction," in Encyclopedia of Separation Science, Vol. 10 (I.D. Wilson, E.R. Adlard, M. Cooke, and C.F. Poole, eds.), London:Academic Press, London, 2000, pp. 4636–4643.
- Colin Poole & Michael Cooke, 2000, Extraction, in Encyclopedia of Separation Science, 10 Vols., ISBN 9780122267703, see , accessed 12 May 2014.
See also
References
- ↑ 1. Liquid-Liquid Extraction Chemistry, LibreTexts. 2013-10-25. Retrieved 2017-03-26
Title: Fundamentals Of Analytical Chemistry 8th Edition, Fundamentals Of Analytical Chemistry 8th Edition
