Rimegepant
Rimegepant (marketed as Nurtec ODT in the United States) is a medication for the treatment of an acute migraine with or without aura in adults.[1] It is not used prophylactically. [2]
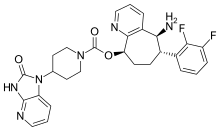 | |
| Clinical data | |
|---|---|
| Trade names | Nurtec ODT |
| Other names | BHV-3000, BMS-927711 |
| License data |
|
| Routes of administration | By mouth |
| Drug class | calcitonin gene-related peptide receptor antagonist |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C28H28F2N6O3 |
| Molar mass | 534.568 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Taken by mouth to dissolve on or under the tongue,[2] rimegepant takes effect within an hour and can provide relief for up to 48 hours. It is not a narcotic and has no addictive potential; thus it is not designated as a controlled substance. It works by blocking CGRP receptors.
Approved for use in the United States in February 2020, rimegepant is produced and marketed by Biohaven Pharmaceuticals.[3][1]
Mechanism of action
Rimegepant is a small molecule calcitonin gene-related peptide (CGRP) receptor antagonist.[4]
History
Originally discovered at Bristol-Myers Squibb,[5] rimegepant was developed by Biohaven Pharmaceuticals, which now markets the drug in the United States after receiving FDA approval in February 2020.[6] Approval was based on evidence from one clinical trial of 1,351 subjects with migraine headaches.[1]
References
- "Drug Trials Snapshots: Nurtec ODT". U.S. Food and Drug Administration (FDA). 27 February 2020. Retrieved 19 March 2020.

- "NURTEC ODT- rimegepant sulfate tablet, orally disintegrating". DailyMed. 19 February 2020. Retrieved 19 March 2020.
- "Nurtec ODT: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Retrieved 28 February 2020.
- Diener HC, Charles A, Goadsby PJ, Holle D (October 2015). "New therapeutic approaches for the prevention and treatment of migraine". The Lancet. Neurology. 14 (10): 1010–22. doi:10.1016/S1474-4422(15)00198-2. PMID 26376968.
- "Rimegepant - Biohaven Pharmaceuticals Holding Company". Adis Insight. Springer Nature Switzerland AG.
- "Rimegepant (BHV-3000) – for acute treatment of Migraine". Biohaven Pharmaceuticals.
External links
- "Rimegepant". Drug Information Portal. U.S. National Library of Medicine.
- Clinical trial number NCT03461757 for "Trial in Adult Subjects With Acute Migraines" at ClinicalTrials.gov