Secondary metabolite
Secondary metabolites also called Specialized Metabolites, secondary products or Natural Products are organic compounds produced by bacteria, fungi, or plants which are not directly involved in the normal growth, development, or reproduction of the organism. Unlike primary metabolites, absence of secondary metabolites does not result in immediate death, but rather in a long-term impairment of the organism's survivability, fecundity, or aesthetics, or perhaps in no significant change at all. Specific secondary metabolites are often restricted to a narrow set of species within a phylogenetic group. Secondary metabolites often play an important role in plant defense against herbivory and other interspecies defenses. Humans use secondary metabolites as medicines, flavourings, pigments, and recreational drugs.[1]
The term secondary metabolite was first coined by Albrecht Kossel, a 1910 Nobel Prize laureate for medicine and physiology in 1910.[2] 30 years later a Polish botanist Friedrich Johann Franz Czapek described secondary metabolites as end products of nitrogen metabolism.[3]
Secondary metabolites aid a host in important functions such as protection, competition, and species interactions, but are not necessary for survival. One important defining quality of secondary metabolites is their specificity. Usually, secondary metabolites are specific to an individual species,[4] though there is considerable evidence that horizontal transfer across species or genera of entire pathways plays an important role in bacterial (and, likely, fungal) evolution.[5] Research also shows that secondary metabolic can affect different species in varying ways. In the same forest, four separate species of arboreal marsupial folivores reacted differently to a secondary metabolite in eucalypts.[6] This shows that differing types of secondary metabolites can be the split between two herbivore ecological niches.[6] Additionally, certain species evolve to resist secondary metabolites and even use them for their own benefit. For example, monarch butterflies have evolved to be able to eat milkweed (Asclepias) despite the toxic secondary metabolite it contains.[7] This ability additionally allows the butterfly and caterpillar to be toxic to other predators due to the high concentration of secondary metabolites consumed.[7]
Categories
Most of the secondary metabolites of interest to humankind fit into categories which classify secondary metabolites based on their biosynthetic origin. Since secondary metabolites are often created by modified primary metabolite synthases, or "borrow" substrates of primary metabolite origin, these categories should not be interpreted as saying that all molecules in the category are secondary metabolites (for example the steroid category), but rather that there are secondary metabolites in these categories.
Plant secondary metabolites
Plants are capable of producing and synthesizing diverse groups of organic compounds and are divided into two major groups: primary and secondary metabolites. Secondary metabolites are metabolic intermediates or products which are not essential to growth and life of the producing plants but rather required for interaction of plants with their environment and produced in response to stress. Their antibiotic, antifungal and antiviral properties protect the plant from pathogens. Some secondary metabolites such as Phenylpropanoid protect plants from UV damage.[8] The biological effects of plant secondary metabolites on humans have been known since ancient times. The herb artemisia annua which contains Artemisinin, has been widely used in Chinese traditional medicine more than two thousand years ago. Plant secondary metabolites are classified by their chemical structure and can be divided into four major classes: terpenes, phenolics, glycosides and alkaloids.[9]

- Terpenes constitute a large class of natural products which are composed of isoprene units. Terpenes are only hydrocarbons and terpenoids are oxygenated hydrocarbons. The general molecular formula of terpenes are multiples of (C5H8)n , where 'n' is number of linked isoprene units. Hence, terpenes are also termed as isoprenoid compounds. Classification is based on the number of isoprene units present in their structure.
| Number of isoprene units | Name | Carbon atoms |
|---|---|---|
| 1 | Hemiterpene | C5 |
| 2 | Monoterpene | C10 |
| 3 | Sesquiterpenes | C15 |
| 4 | Diterpene | C20 |
| 5 | Sesterterpene | C25 |
| 6 | Triterpene | C30 |
| 7 | Sesquarterterpene | C35 |
| 8 | Tetraterpene | C40 |
| More than 8 | Polyterpene |
- A phenolic is a chemical compound characterized by the presence of aromatic ring structure bearing one or more hydroxyl groups. Phenolics are most abundant secondary metabolites of plants ranging from simple molecules such as phenolic acid to highly polymerized substances such as tannins. Classes of phenolics have been characterized on the basis of their basic skeleton.
| No. of carbon atoms | Basic skeleton | Class |
|---|---|---|
| 6 | C6 | Simple phenols |
| 7 | C6 - C1 | Phenolic acids |
| 8 | C6 - C2 | Acetophenone, Phenyle acetic acid |
| 9 | C6 - C3 | Phenylepropanoids, hydroxycinnamic acid, coumarins |
| 10 | C6 - C4 | Naphthoquinone |
| 13 | C6 - C1- C6 | Xanthone |
| 14 | C6 - C2 - C6 | Stilbene, anthraquinone |
| 15 | C6 - C3 - C6 | Flavonoids, isoflavanoids |
| 18 | (C6 - C3 ) 2 | lignans, neolignans |
| 30 | ( C6 - C3 - C6)2 | Biflavonoids |
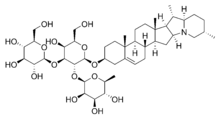
- A glycoside is a molecule in which carbohydrate is bound by a glycosidic bond to a non-carbohydrate moiety containing a hydroxyl group. The sugar most commonly found in glycosides is glucose. Among the diverse group of glycosides, three are most important. These are saponins, cardiac glycosides and cyanogenic glycosides.
- Alkaloids: are a diverse group of nitrogen-containing basic compounds. They are typically derived from plant sources and contain one or more nitrogen atoms. Chemically they are very heterogeneous. Based on chemical structures, they may be classified into two broad categories:
- Non heterocyclic or atypical alkaloids, for example hordenine or N-methyltyramine, colchicine, and taxol
- Heterocyclic or typical alkaloids, for example quinine, caffeine, and nicotine
Microbial secondary metabolites
Bacterial secondary metabolites
Bacterial production of secondary metabolites starts in the stationary phase as a consequence of lack of nutrients or in response to environmental stress. Secondary metabolite synthesis in. bacteria is not essential for their growth, however, they allow them to better interact with their ecological niche. The main synthetic pathways of secondary metabolite production in bacteria are; b-lactam, oligosaccharide, shikimate, polyketide and non-ribosomal pathways.[10] Many bacterial secondary metabolites are toxic to mammals. When secreted those poisonous compounds are known as exotoxins whereas those found in the prokaryotic cell wall are endotoxins.
An example of a bacterial secondary metabolite with a positive and negative effect on humans is botulinum toxin synthesised by clostridium botulinum. This Exotoxin often builds up in incorrectly canned foods and when ingested blocks cholinergic neurotransmission leading to muscle paralysis or death. However, botulinum toxin also has multiple medical uses such as treatment of muscle spasticity, migraine and cosmetics use.
Fungal secondary metabolites
The three main classes of fungal secondary metabolites are: polyketides, nonribosomal peptides and terpenes. Although fungal SMs are not required for growth they play an essential role in survival of fungi in their ecological niche.[11] The most known fungal secondary metabolite is penicillin discovered by Alexander Fleming in 1928. Later in 1945, Fleming alongside Ernst Chain and Howard Florey received a Nobel Prize for its discovery which was pivotal in reducing the number of deaths in WWII by over 100000.[12]
Lovastatin was the first FDA approved secondary metabolite to lower cholesterol levels. Lovastatin occurs naturally in low concentrations in oyster mushrooms,[13] red yeast rice,[14] and Pu-erh.[15] Lovastatin's Mode of action is competitive inhibition of HMG-CoA reductase, and a rate-limiting enzyme responsible for converting HMG-Coa to mevalonate.
Fungal Secondary metabolites are also known to be dangerous to humans. Claviceps purpurea, a member of the Ergot group of fungi typically growing on rye, results in death when ingested. The build up of poisonous alkaloids found in Claviceps purpurea lead to symptoms such as seizures and spasms, diarrhea, paresthesias, Itching, psychosis or gangrene. Currently removal of ergot bodies requires putting the rye in brine solution with healthy grains sinking and infected floating.[16]
Human health implications
Most polyphenol nutraceuticals from plant origin must undergo intestinal transformations, by microbiota and enterocyte enzymes, in order to be absorbed at enterocyte and colonocyte levels. This gives rise to diverse beneficial effects in the consumer, including a vast array of protective effects against viruses, bacteria, and protozoan parasites.[17]
Secondary metabolites also have a strong impact on the food humans eat. Some researchers believe that certain secondary metabolite volatiles are responsible for human food preferences that may be evolutionarily based in nutritional food.[18] This area of interest has not been thoroughly researched, but has interesting implications for human preference. Many secondary metabolites aid the plant in gaining essential nutrients, such as nitrogen. For example, legumes use flavonoids to signal a symbiotic relationship with nitrogen fixing bacteria (rhizobium) to increase their nitrogen uptake.[7] Therefore, many plants that utilize secondary metabolites are high in nutrients and advantageous for human consumption.
Plant secondary metabolites in medicine
Many drugs used in modern medicine are derived from plant secondary metabolites.

The two most commonly known Terpenoids are Artemisinin and Taxol®. Artemisinin was widely used in Traditional Chinese medicine and later rediscovered as a powerful antimalarial by a Chinese scientist Tu Youyou. She was later awarded the Nobel Prize for the discovery in 2015. Currently, the malaria parasite, Plasmodium falciparum, has become resistant to artemisinin alone and the World Health Organization recommends its use with other antimalarial drugs for a successful therapy. Paclitaxel the active compound found in Taxol is a chemotherapy drug used to treat many forms of cancers including ovarian cancer, breast cancer, lung cancer, Kaposi sarcoma, cervical cancer, and pancreatic cancer.[19] Taxol was first isolated in 1973 from barks of a coniferous tree, the Pacific Yew.[20]
Morphine and Codeine both belong to the class of alkaloids and are derived from Opium poppies. Morphine was discovered in 1804 by a German pharmacist Friedrich Sertürnert. It was the first active alkaloid extracted from the opium poppy. It is mostly known for its strong analgesic effects, however, morphine is also used to treat shortness of breath and treatment of addiction to stronger opiates such as heroin.[21][22] Despite its positive effects on humans, morphine has very strong adverse effects, such as addiction, hormone imbalance or constipation.[22][23] Due to its highly addictive nature morphine is a strictly controlled substance around the world, used only in very severe cases with some countries underusing it compared to the global average due the social stigma around it.[24]

Codeine, also an alkaloid derived from the opium poppy, is considered the most widely used drug in the world according to World Health Organization. It was first isolated in 1832 by a French chemist Pierre Jean Robiquet, also known for the discovery of caffeine and a widely used red dye alizarin.[26] Primarily codeine is used to treat mild pain and relief coughing[27] although in some cases it is used to treat diarrhea and some forms of Irritable bowel syndrome.[27] Codeine has the strength of 0.1-0.15 compared to morphine ingested orally,[28] hence it is much safer to use. Although codeine can be extracted from the opium poppy, the process is not feasible economically due to the low abundance of pure codeine in the plant. A chemical process of methylation of the much more abundant morphine is the main method of production.[29]
Atropine is an alkaloid first found in atropa belladonna, a member of the nightshade family. Pure atropine was first extracted in the 19th century, however its' medical use dates back to fourth century B.C. where it was used for wounds, gout, and sleeplessness. Currently atropine is administered intraveinously to treat bradycardia and as an antidote to organophosphate poisoning. Overdosing of atropine may lead to atropine poisoning which results in side effect such as Blurred vision, nausea, lack of sweating, dry mouth and tachycardia.[30]
Resveratrol is a flavinoid belonging to the phenolics family. It is highly abundant in grapes, blueberries, raspberries and peanuts. It is commonly used as a dietary supplement for extending life and reducing the risk of cancer and heart disease, however no there is no strong evidence supporting that.[31][32] Despite no strong evidence for the use of resveratrol flavonoids on the whole have beneficial effects for humans. For instance certain studies shown that flavonoids have direct antibiotic activity.[33] A number of in vitro and limited in vivo studies shown that flavonoids such as Quercetin have synergistic activity with antibiotics and are able to suppress bacterial loads.[34]
Digoxin is a cardiac glycoside first derived by William Withering in 1785 from the Fox Glove plant. It is typically used to treat heart conditions such as atrial fibrillation, atrial flutter or heart failure.[35] Digoxin can, however, have side effects such as nausea, bradycardia, diarrhea or even life threatening arrhythmia.
Biotechnological approaches

Selective breeding was used as one of the first biotechnological techniques used to reduce the unwanted secondary metabolites in food, such as naringin causing bitterness in grapefruit.[36] In some cases increasing the content of secondary metabolites in a plant is the desired outcome. Traditionally this was done using in-vitro plant tissue culture techniques which allow for: control of growth conditions, mitigate seasonality of plants or protect them from parasites and harmful-microbes. Synthesis of secondary metabolites can be further enhanced by introducing elicitors into a tissue plant culture, such as jasmonic acid, UV-B or ozone. These compounds induce stress onto a plant leading to increased production of secondary metabolites.
To further increase the yield of SMs new approaches have been developed. A novel approach used by Evolva uses recombinant yeast S.cervisiae strains to produce secondary metabolitersnormally found in plants. The first successful chemical compound synthesised with Evolva was vanillin, widely used in the food beverage industry as flavouring. The process involves inserting the desired secondary metabolite gene into an artificial chromosome in the recombinant yeast leading to synthesis of vanillin. Currently Evolva produces a wide array of chemicals such as stevia, resveratrol or nootkatone.
Nagoya protocol
With the development of recombinant technologies the Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from their Utilization to the Convention on Biological Diversity was signed in 2010. The protocol regulates the conservation and protection of genetic resources to prevent the exploitation of smaller and poorer countries. If genetic, protein or small molecule resources sourced from biodiverse countries become profitable a compensation scheme was put in place for the countries of origin.[37]
List of known secondary metabolites
Small "small molecules"
- Alkaloids (usually a small, heavily derivated amino acid):
- Hyoscyamine, present in Datura stramonium
- Atropine, present in Atropa belladonna, Deadly nightshade
- Cocaine, present in Erythroxylum coca the Coca plant
- Scopolamine, present in the Solanaceae (nightshade) plant family
- Codeine and Morphine, present in Papaver somniferum, the opium poppy
- Tetrodotoxin, a microbial product in Fugu and some salamanders
- Vincristine & Vinblastine, mitotic inhibitors found in the Rosy Periwinkle
- Terpenoids (come from semiterpene oligomerization):
- Azadirachtin, (Neem tree)
- Artemisinin, present in Artemisia annua Chinese wormwood
- tetrahydrocannabinol, present in cannabis
- Steroids (Terpenes with a particular ring structure)
- Saponins (plant steroids, often glycosylated)
- Flavonoids (or bioflavonoids) (from the Latin word flavus meaning yellow, their color in nature) are a class of plant and fungus secondary metabolites):
- Glycosides (heavily modified sugar molecules):
- Natural phenols:
- Phenazines:
- Pyocyanin
- Phenazine-1-carboxylic acid (and derivatives)
- Biphenyls and dibenzofurans are phytoalexins of the Pyrinae[38]
Big "small molecules", produced by large, modular, "molecular factories"
- Polyketides:
- Erythromycin
- Lovastatin and other statins
- Discodermolide
- Aflatoxin B1
- Avermectins
- Nystatin
- Rifamycin
- Fatty acid synthase products :
- Jawsamycin (FR-900848)
- U-106305
- phloroglucinols
- Nonribosomal peptides:
- Ribosomally synthesized and post-translationally modified peptides:
- Hybrids of the above three:
- Polyphenols
Non-"small molecules": DNA, RNA, ribosome, or polysaccharide "classical" biopolymers
- Ribosomal peptides:
- Microcin-J25
See also
- Secondary metabolism
- Hairy root culture, a strategy used in plant tissue culture to produce commercially viable quantities of valuable secondary metabolites
- Metabolite
- Primary metabolite
- Plant physiology
References
- "Secondary metabolites - Knowledge Encyclopedia". www.biologyreference.com. Retrieved 2016-05-10.
- Jones, Mary Ellen (September 1953). "Albrecht Kossel, A Biographical Sketch". The Yale Journal of Biology and Medicine. 26 (1): 80–97. PMC 2599350. PMID 13103145.
- Bourgaud, F.; Gravot, A.; Milesi, S.; Gontier, E. (1 October 2001). "Production of plant secondary metabolites: a historical perspective". Plant Science. 161 (5): 839–851. doi:10.1016/S0168-9452(01)00490-3.
- Pichersky E, Gang DR (October 2000). "Genetics and biochemistry of secondary metabolites in plants: an evolutionary perspective". Trends in Plant Science. 5 (10): 439–45. doi:10.1016/S1360-1385(00)01741-6. PMID 11044721.
- Juhas M, van der Meer JR, Gaillard M, Harding RM, Hood DW, Crook DW (March 2009). "Genomic islands: tools of bacterial horizontal gene transfer and evolution". FEMS Microbiology Reviews. 33 (2): 376–93. doi:10.1111/j.1574-6976.2008.00136.x. PMC 2704930. PMID 19178566.
- Jensen LM, Wallis IR, Marsh KJ, Moore BD, Wiggins NL, Foley WJ (September 2014). "Four species of arboreal folivore show differential tolerance to a secondary metabolite". Oecologia. 176 (1): 251–8. Bibcode:2014Oecol.176..251J. doi:10.1007/s00442-014-2997-4. PMID 24974269.
- Croteau R, Kutchan TM, Lewis NG (2012-07-03). "Chapter 24: Natural products (secondary metabolites)". In Civjan N (ed.). Natural products in chemical biology. Hoboken, New Jersey: Wiley. pp. 1250–1319. ISBN 978-1-118-10117-9.
- Korkina, Liudmila; Kostyuk, Vladimir; Potapovich, Alla; Mayer, Wolfgang; Talib, Nigma; De Luca, Chiara (2 May 2018). "Secondary Plant Metabolites for Sun Protective Cosmetics: From Pre-Selection to Product Formulation". Cosmetics. 5 (2): 32. doi:10.3390/cosmetics5020032.
- Pranav Kumar. (2013). Life Sciences : Fundamentals and practice. Mina, Usha. (3rd ed.). New Delhi: Pathfinder Academy. ISBN 9788190642774. OCLC 857764171.
- Gokulan, Kuppan; Khare, Sangeeta; Cerniglia, C. (2014-12-31), "Metabolic Pathways: Production of Secondary Metabolites of Bacteria", Encyclopedia of Food Microbiology, pp. 561–569, ISBN 978-0-12-384733-1, retrieved 2020-04-10
- Boruta, Tomasz (2018-01-01). "Uncovering the repertoire of fungal secondary metabolites: From Fleming's laboratory to the International Space Station". Bioengineered. 9 (1): 12–16. doi:10.1080/21655979.2017.1341022. ISSN 2165-5979. PMC 5972916. PMID 28632991.
- Conniff, Richard (2017-07-03). "Penicillin: Wonder Drug of World War II". HistoryNet. Retrieved 2020-04-11.
- Gunde-Cimerman, N; Cimerman, A (March 1995). "Pleurotus fruiting bodies contain the inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase-lovastatin". Experimental Mycology. 19 (1): 1–6. doi:10.1006/emyc.1995.1001. PMID 7614366.
- Liu J, Zhang J, Shi Y, Grimsgaard S, Alraek T, Fønnebø V (2006). "Chinese red yeast rice (Monascus purpureus) for primary hyperlipidemia: a meta-analysis of randomized controlled trials". Chin Med. 1 (1): 4. doi:10.1186/1749-8546-1-4. PMC 1761143. PMID 17302963.
- Zhao ZJ, Pan YZ, Liu QJ, Li XH (2013). "Exposure assessment of lovastatin in Pu-erh tea". International Journal of Food Microbiology. 164 (1): 26–31. doi:10.1016/j.ijfoodmicro.2013.03.018. PMID 23587710.
- Uys, H.; Berk, M. (June 1996). "A controlled double blind study of zuclopenthixol acetate compared with clothiapine in acute psychosis including mania and exacerbation of chronic psychosis". European Neuropsychopharmacology. 6: 60. doi:10.1016/0924-977x(96)87580-8. ISSN 0924-977X.
- Marín L, Miguélez EM, Villar CJ, Lombó F (6 April 2018). "Bioavailability of dietary polyphenols and gut microbiota metabolism: antimicrobial properties". BioMed Research International. 2015: 905215. doi:10.1155/2015/905215. PMC 4352739. PMID 25802870.
- Goff SA, Klee HJ (February 2006). "Plant volatile compounds: sensory cues for health and nutritional value?". Science. 311 (5762): 815–9. Bibcode:2006Sci...311..815G. doi:10.1126/science.1112614. PMID 16469919.
- "Paclitaxel Monograph for Professionals". Drugs.com. Retrieved 2020-04-04.
- "Success Story: Taxol". dtp.cancer.gov. Retrieved 2020-04-04.
- Da, Mahler; Pa, Selecky; Cg, Harrod; Jo, Benditt; V, Carrieri-Kohlman; Jr, Curtis; Hl, Manning; Ra, Mularski; B, Varkey (March 2010). "American College of Chest Physicians Consensus Statement on the Management of Dyspnea in Patients With Advanced Lung or Heart Disease". Chest. 137 (3): 674–91. doi:10.1378/chest.09-1543. PMID 20202949.
- Kastelic, Andrej; Dubajic, Goran; Strbad, Ervin (November 2008). "Slow-release oral morphine for maintenance treatment of opioid addicts intolerant to methadone or with inadequate withdrawal suppression". Addiction (Abingdon, England). 103 (11): 1837–1846. doi:10.1111/j.1360-0443.2008.02334.x. ISSN 1360-0443. PMID 19032534.
- A, Calignano; S, Moncada; M, Di Rosa (1991-12-16). "Endogenous Nitric Oxide Modulates Morphine-Induced Constipation". Biochemical and Biophysical Research Communications. 181 (2): 889–93. doi:10.1016/0006-291x(91)91274-g. PMID 1755865.
- Manjiani, Deepak; Paul, D. Baby; Kunnumpurath, Sreekumar; Kaye, Alan David; Vadivelu, Nalini (2014). "Availability and Utilization of Opioids for Pain Management: Global Issues". The Ochsner Journal. 14 (2): 208–215. ISSN 1524-5012. PMC 4052588. PMID 24940131.
- "Wayback Machine". 2007-09-28. Archived from the original on 2007-09-28. Retrieved 2020-04-11.
- Wisniak, Jaime (2013-03-01). "Pierre-Jean Robiquet". Educación Química. 24: 139–149. doi:10.1016/S0187-893X(13)72507-2. ISSN 0187-893X.
- "Codeine Monograph for Professionals". Drugs.com. Retrieved 2020-04-05.
- "Equianalgesic", Wikipedia, 2020-04-02, retrieved 2020-04-05
- "UNODC - Bulletin on Narcotics - 1958 Issue 3 - 005". United Nations : Office on Drugs and Crime. Retrieved 2020-04-05.
- "Atropine Side Effects Center".
- "Resveratrol: MedlinePlus Supplements". medlineplus.gov. Retrieved 2020-04-07.
- Vang, Ole; Ahmad, Nihal; Baile, Clifton A.; Baur, Joseph A.; Brown, Karen; Csiszar, Anna; Das, Dipak K.; Delmas, Dominique; Gottfried, Carmem; Lin, Hung-Yun; Ma, Qing-Yong (2011-06-16). "What Is New for an Old Molecule? Systematic Review and Recommendations on the Use of Resveratrol". PLOS ONE. 6 (6): e19881. Bibcode:2011PLoSO...619881V. doi:10.1371/journal.pone.0019881. ISSN 1932-6203. PMC 3116821. PMID 21698226.
- Cushnie, T. P. Tim; Lamb, Andrew J. (November 2005). "Antimicrobial activity of flavonoids". International Journal of Antimicrobial Agents. 26 (5): 343–356. doi:10.1016/j.ijantimicag.2005.09.002. ISSN 0924-8579. PMC 7127073. PMID 16323269.
- Panche, A. N.; Diwan, A. D.; Chandra, S. R. (2016-12-29). "Flavonoids: an overview". Journal of Nutritional Science. 5: e47. doi:10.1017/jns.2016.41. ISSN 2048-6790. PMC 5465813. PMID 28620474.
- "Digoxin Monograph for Professionals". Drugs.com. Retrieved 2020-04-07.
- Drewnowski, Adam; Gomez-Carneros, Carmen (2000-12-01). "Bitter taste, phytonutrients, and the consumer: a review". The American Journal of Clinical Nutrition. 72 (6): 1424–1435. doi:10.1093/ajcn/72.6.1424. ISSN 0002-9165. PMID 11101467.
- Unit, Biosafety (2020-04-14). "The Nagoya Protocol on Access and Benefit-sharing". www.cbd.int. Retrieved 2020-04-15.
- Chizzali C, Beerhues L (2012). "Phytoalexins of the Pyrinae: Biphenyls and dibenzofurans". Beilstein Journal of Organic Chemistry. 8: 613–20. doi:10.3762/bjoc.8.68. PMC 3343287. PMID 22563359.
External links
