Terazosin
Terazosin, sold under the brand name Hytrin among others, is a medication used to treat symptoms of an enlarged prostate and high blood pressure.[1] For high blood pressure, it is a less preferred option.[1] It is taken by mouth.[1]
 | |
| Clinical data | |
|---|---|
| Trade names | Hytrin, Zayasel, others |
| Other names | [4-(4-amino-6,7-dimethoxy-quinazolin-2-yl)piperazin-1-yl]-tetrahydrofuran-2-yl-methanone |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a693046 |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 90-94% |
| Elimination half-life | 12 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.118.191 |
| Chemical and physical data | |
| Formula | C19H25N5O4 |
| Molar mass | 387.440 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Common side effects include dizziness, headache, tiredness, swelling, nausea, and low blood pressure with standing.[1] Severe side effects may include priapism and low blood pressure.[1] Prostate cancer should be ruled out before starting treatment.[1] It is an alpha-1 blocker and works by relaxing blood vessels and the opening of the bladder.[1]
Terazosin was patented in 1975 and came into medical use in 1985.[2] It is available as a generic medication.[3] A month supply in the United Kingdom costs the NHS less than £2 as of 2019.[3] In the United States the wholesale cost of this amount is about US$4.50.[4] In 2017, it was the 194th most commonly prescribed medication in the United States, with more than two million prescriptions.[5][6]
Formulations
It is available in 1 mg, 2 mg, 5 mg or 10 mg doses.[7]
Synthesis
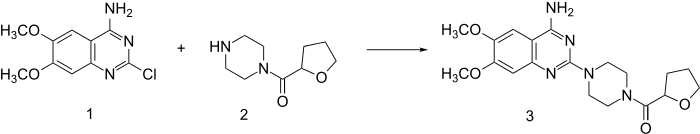
Reaction of piperazine with 2-furoyl chloride followed by catalytic hydrogenation of the furan ring leads to 2. This, when heated in the presence of 2-chloro-6,7-dimethoxyquinazolin-4-amine (1) undergoes direct alkylation to terazosin (3).
References
- "Terazosin Hydrochloride Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 17 March 2019.
- Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 455. ISBN 9783527607495.
- British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 768. ISBN 9780857113382.
- "NADAC". Centers for Medicare and Medicaid Services. 27 February 2019.
- "The Top 300 of 2020". ClinCalc. Retrieved 11 April 2020.
- "Terazosin - Drug Usage Statistics". ClinCalc. Retrieved 11 April 2020.
- "Terazosin Hydrochloride Capsule". DailyMed. National Library of Medicine, National Institutes of Health.
- US 4026894, Winn M, Kyncl J, Dunnigan DA, Jones PH, issued 31 May 1977, assigned to Abbott