Pentylenetetrazol
 | |
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard |
100.000.200 |
| Chemical and physical data | |
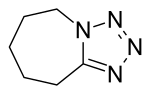
| Formula | C6H10N4 |
| Molar mass | 138.171 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Pentylenetetrazol, also known as pentylenetetrazole, metrazol, pentetrazol (INN), pentamethylenetetrazol, Corazol, Cardiazol, deumacard or PTZ, is a drug formerly used as a circulatory and respiratory stimulant. High doses cause convulsions, as discovered by the Hungarian-American neurologist and psychiatrist Ladislas J. Meduna in 1934. It has been used in convulsive therapy, and was found to be effective—primarily for depression—but side-effects such as uncontrolled seizures were difficult to avoid.[1] In 1939 pentylenetetrazol was replaced by electroconvulsive therapy, easier to administer, as the preferred method for inducing seizures in England's mental hospitals. In the US its approval by the FDA was revoked in 1982.[2] It was used until recently in Italy as a cough suppressant.[3]
Mechanism
The mechanism of pentylenetetrazol is not well understood, and it may have multiple mechanisms of action. In 1984, Squires et al. published a report analyzing pentylenetetrazol and several structurally related convulsant drugs. They found that in vivo convulsant potency was strongly correlated to in vitro affinity to the picrotoxin binding site on the GABA-A receptor complex. Many GABA-A ligands are effective anticonvulsants, such as the sedatives diazepam and phenobarbital, but presumably pentylenetetrazol has the opposite effect when it binds to the GABA-A receptor.[4]
Several studies have focused on the way pentylenetetrazol influences neuronal ion channels. A 1987 study found that pentylenetetrazol increases calcium influx and sodium influx, both of which depolarize the neuron. Because these effects were antagonized by calcium channel blockers, it was concluded that pentylenetetrazol acts at calcium channels, and it causes calcium channels to lose selectivity and conduct sodium ions as well.[5]
cAMP dependent mechanism
One study assessed the effect of cAMP, its analogs and dependent protein kinase on pentylenetetrazole-induced seizure in vivo. The finding show that cAMP analog, as well as phosphodiesterase and protein kinase inhibitors affected the epileptogenic activity of pentylenetetrazole. This finding shows the involvement of cAMP, its downstream and upstream on pentylenetetrazole activity.[6]
Uses
Pentylenetetrazol has been used experimentally to study seizure phenomena and to identify pharmaceuticals that may control seizure susceptibility. Pentylenetetrazol is also a prototypical anxiogenic drug and, has been extensively utilized in animal models of anxiety. Pentylenetetrazol produces a reliable discriminative stimulus which is largely mediated by the GABAA receptor. Several classes of compounds can modulate the pentylenetetrazol discriminative stimulus including 5-HT1A, 5-HT3, NMDA, glycine, and L-type calcium channel ligands.[7]
Stanford University researchers proposed PTZ as a candidate for pharmacological treatment of Down syndrome. A brief communication in the April 2007 issue of Nature Neuroscience outlined an experiment designed to test the underlying theory proposed to explain the purported efficacy of GABAA antagonists in restoring the declarative memory deficits associated with the mouse model of human Down Syndrome. Ts65Dn mice injected with a 2-week regimen of either of two compounds picrotoxin or bilobalide (both GABA antagonists) showed marked improvements in both exploration and recognition of novel objects over controls injected with only saline. These results were duplicated in a second experiment with mice fed either plain milk or a combination of milk and a non-epileptogenic dose of PTZ daily for 17 days. PTZ-fed mice achieved novel object task scores comparable to wild-type (normal) mice. These improvements persisted at least 1 to 2 months after the treatment regimen. Not surprisingly these compounds' efficacies were accompanied by the normalization of long-term potentiation in the dentate gyrus one month after the end of treatment, further suggesting persistent drug-mediated improvements in learning and memory.[8]
The finding of pentylenetetrazol's effectiveness in treating a mouse model of Down syndrome has led to it being explored as a means of correcting other learning deficiencies. Specifically, hamsters denied their natural circadian rhythm (though not denied sleep) had their memory restored to near-normal levels when treated with pentylenetetrazol.[9]
A study found that the phytocannabinoid THC protect mice from more severe cognitive deficits induced by pentylenetetrazole, and suggest that a pre- or post-conditioning treatment with extremely low doses of THC, several days before or after brain injury, may provide safe and effective long-term neuroprotection.[10]
Mention in literature
Allen Ginsberg mentioned Metrazol in his poem "Kaddish": No love since Naomi screamed—since 1923?—now lost in Greystone ward—new shock for her—Electricity, following the 40 Insulin. And Metrazol had made her fat.
See also
References
- ↑ Read, Charles F. (1940). "Consequences of metrazol shock therapy". American Journal of Psychiatry. 97 (3): 667–76. doi:10.1176/ajp.97.3.667.
- ↑ Minkel JR (February 25, 2007). "Drug May Counteract Down Syndrome". Scientific American. Retrieved 2007-03-20.
- ↑ Torrinomedica (July 2016). "Cardiazol Paracodina". Torrinomedica s.r.l.
- ↑ Squires RF, Saederup E, Crawley JN, Skolnick P, Paul SM (1984). "Convulsant potencies of tetrazoles are highly correlated with actions on GABA / benzodiazepine / picrotoxin receptor complexes in brain". Life Sci. 35 (14): 1439–44. doi:10.1016/0024-3205(84)90159-0. PMID 6090836.
- ↑ Papp A, Fehér O, Erdélyi L (1987). "The ionic mechanism of the pentylenetetrazol convulsions". Acta Biol. Hung. 38 (3–4): 349–61. PMID 3503442.
- ↑ Hosseini-Zare MS, Salehi F, Seyedi SY, Azami K, Ghadiri T, Mobasseri M, Gholizadeh S, Beyer C, Sharifzadeh M (2011). "Effects of pentoxifylline and H-89 on epileptogenic activity of bucladesine in pentylenetetrazol-treated mice". European Journal of Pharmacology. 670 (2–3): 464–70. doi:10.1016/j.ejphar.2011.09.026. PMID 21946102.
- ↑ Jung ME, Lal H, Gatch MB (2002). "The discriminative stimulus effects of pentylenetetrazol as a model of anxiety: recent developments". Neurosci. Biobehav. Rev. 26 (4): 429–39. doi:10.1016/S0149-7634(02)00010-6. PMID 12204190.
- ↑ Fernandez F, Morishita W, Zuniga E, Nguyen J, Blank M, Malenka RC, Garner CC (2007). "Pharmacotherapy for cognitive impairment in a mouse model of Down syndrome" (PDF). Nat. Neurosci. 10 (4): 411–3. doi:10.1038/nn1860. PMID 17322876. Archived from the original (PDF) on 2011-07-20.
- ↑ Ruby NF, Hwang CE, Wessells C, Fernandez F, Zhang P, Sapolsky R, Heller HC (2008). "Hippocampal-dependent learning requires a functional circadian system". PNAS USA. 105 (40): 15593–8. doi:10.1073/pnas.0808259105. PMC 2563080. PMID 18832172.
- ↑ "Pre- and post-conditioning treatment with an ultra-low dose of Δ9-tetrahydrocannabinol (THC) protects against pentylenetetrazole (PTZ)-induced cognitive damage". Behavioural Brain Research. 220 (1): 194–201. 2011-06-20. doi:10.1016/j.bbr.2011.02.005. ISSN 0166-4328.