Pyrazolone
Pyrazolone is 5-membered heterocycle containing 2 adjacent nitrogen atoms. It can be viewed as a derivative of pyrazole possessing an additional keto (=O) group.
Structure and synthesis
Pyrazolone can exist in 3 isomers: 3-pyrazolone, 4-pyrazolone, and 5-pyrazolone. These isomers can interconvert via lactam–lactim and imine–enamine tautomerism; these conversion often display photochromism. For pyrazolone derivatives, the 5-pyrazolone form can be stabilized with N-alkyl or N-aryl substituents.
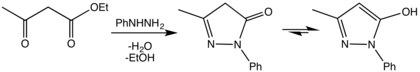
The synthesis of pyrazolones was first reported in 1883 by Ludwig Knorr, via a condensation reaction between ethyl acetoacetate and phenylhydrazine.[1]
Applications
Pharmaceuticals

Pyrazolones are amongst the oldest synthetic pharmaceuticals, starting with the introduction of antipyrine (phenazone) in 1880's.[2][3] The compounds generally act as analgesics and include dipyrone (Metamizole), aminopyrine, ampyrone, famprofazone, morazone, nifenazone, piperylon and propyphenazone. Of these dipyrone is perhaps the most widely used.[2]
Dyes
Pyrazolone groups are present in several important dyes. They are commonly used in combination with azo groups to give a sub-family of azo dyes; sometimes referred to as azopyrazolones (tartrazine, orange B, mordant red 19, yellow 2G).
Ligands
Pyrazolones have been studied as ligands.[4]
References
- ↑ Knorr, Ludwig (July 1883). "Einwirkung von Acetessigester auf Phenylhydrazin". Berichte der deutschen chemischen Gesellschaft (in German). 16 (2): 2597–2599. doi:10.1002/cber.188301602194.
- 1 2 Brogden, Rex N. (1986). "Pyrazolone Derivatives". Drugs. 32 (Supplement 4): 60–70. doi:10.2165/00003495-198600324-00006.
- ↑ Brune, Kay (December 1997). "The early history of non-opioid analgesics". Acute Pain. 1 (1): 33–40. doi:10.1016/S1366-0071(97)80033-2.
- ↑ CASAS, J; GARCIATASENDE, M; SANCHEZ, A; SORDO, J; TOUCEDA, A (June 2007). "Coordination modes of 5-pyrazolones: A solid-state overview". Coordination Chemistry Reviews. 251 (11–12): 1561–1589. doi:10.1016/j.ccr.2007.02.010.
External links
- Pyrazolones at the US National Library of Medicine Medical Subject Headings (MeSH)
- Pubchem - 3-Pyrazolone
- Pubchem - 5-Pyrazolone