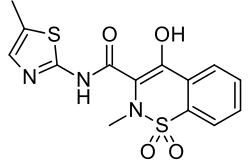
Meloxicam
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Mobic, Metacam |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601242 |
| Pregnancy category | |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 89%[1] |
| Protein binding | 99.4%[1] |
| Metabolism | Hepatic (CYP2C9 and 3A4-mediated)[1] |
| Elimination half-life | 20 hours[1] |
| Excretion | Urine and faeces equally[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| ECHA InfoCard |
100.113.257 |
| Chemical and physical data | |
| Formula | C14H13N3O4S2 |
| Molar mass | 351.403 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Meloxicam is a nonsteroidal anti-inflammatory drug (NSAID) with analgesic and anti-pyretic effects. It is an oxicam closely related to piroxicam, and falls in the enolic acid group of NSAIDs.[2] It was developed by Boehringer-Ingelheim. Meloxicam starts to relieve pain about 30–60 minutes after administration.[3]
As of 2015 the cost for a typical month of medication in the United States is less than $25 USD.[4]
Adverse effects
Meloxicam use can result in gastrointestinal toxicity and bleeding, headaches, rash, and very dark or black stool (a sign of intestinal bleeding). Like other NSAIDs, its use is associated with an increased risk of cardiovascular events such as heart attack and stroke.[5] It has fewer gastrointestinal side effects than diclofenac,[6] piroxicam,[7] naproxen,[8] and perhaps all other NSAIDs which are not COX-2 selective.[6] Although meloxicam inhibits formation of thromboxane A, it does not appear to do so at levels that would interfere with platelet function.
A pooled analysis of randomized, controlled studies of meloxicam therapy of up to 60 days duration found that meloxicam was associated with a statistically significantly lower number of thromboembolic complications than the NSAID diclofenac (0.2% versus 0.8% respectively) but a similar incidence of thromboembolic events to naproxen and piroxicam.[9]
Cardiovascular side effects
Persons with hypertension, high cholesterol, or diabetes are at risk for cardiovascular side effects. Persons with family history of heart disease, heart attack, or stroke must tell their treating physician as the potential for serious cardiovascular side effects is significant.[10][11]
Mechanism of action
Meloxicam blocks cyclooxygenase (COX), the enzyme responsible for converting arachidonic acid into prostaglandin H2—the first step in the synthesis of prostaglandins, which are mediators of inflammation. Meloxicam has been shown, especially at its low therapeutic doses, selectively to inhibit COX-2 over COX-1.[1]
Meloxicam concentrations in synovial fluid range from 40% to 50% of those in plasma. The free fraction in synovial fluid is 2.5 times higher than in plasma, due to the lower albumin content in synovial fluid as compared to plasma. The significance of this penetration is unknown,[2] but it may account for the fact that it performs exceptionally well in treatment of arthritis in animal models.[12]
Veterinary use
Meloxicam is used in veterinary medicine, most commonly in dogs and cats, but also sees off-label use in other animals such as cattle and exotics.[13][14]
Side effects in animals are similar to those found in humans; the principal side effect is gastrointestinal irritation (vomiting, diarrhea, and ulceration). Rarer but important side effects include liver and kidney toxicity.
In healthy dogs given meloxicam, no perioperative adverse effects on the cardiovascular system have been reported at recommended dosages.[15] Perioperative administration of meloxicam to cats did not affect postoperative respiratory rate nor heart rate.[16]
A peer-reviewed journal article cites NSAIDs, including meloxicam, as causing gastrointestinal upset and, at high doses, acute renal failure and CNS signs such as seizures and comas in cats. It adds that cats have a low tolerance for NSAIDs.[17][18]
Meloxicam has been investigated as an alternative to diclofenac by the RSPB to prevent deaths of vultures.
Pharmacokinetics
In dogs, the absorption of meloxicam from the stomach is not affected by the presence of food,[19] with the peak concentration (Cmax) of meloxicam occurring in the blood 7–8 hours after administration.[19] The half-life of meloxicam is approximately 24 hours in dogs.[19]
In the koala (Phascolarctos cinereus), very little meloxicam is absorbed into the blood after oral administration (that is, it has poor bioavailability).[20]
Legal status
United States
Since 2003, meloxicam has been approved in the U.S. for use in dogs for the management of pain and inflammation associated with osteoarthritis, as an oral (liquid) formulation of meloxicam.[21] In January 2005, the product insert added a warning in bold-face type: "Do not use in cats."[22] An injectable formulation for use in dogs was approved by the FDA in November 2003.[23]
In October 2004, a formulation for use in cats was approved for use prior to surgery only.[24] This is an injectable meloxicam, indicated for as a single, one-time dose only, with specific and repeated warnings not to administer a second dose.[25]
In 2005, the U.S. Food and Drug Administration sent a Notice of Violation to the manufacturer for its promotional materials which included promotion of the drug for off-label use.[26]
European Union
In Europe, where the product has been available since the early 1990s, it is licensed for other anti-inflammatory benefits including relief from both acute and chronic pain in dogs. In June 2007, an oral version of meloxicam was licensed for the long-term relief of pain in cats. Meloxicam is also licensed for use in horses, to relieve the pain associated with musculoskeletal disorders.[27]
Other countries
As of June 2008, meloxicam is registered for long-term use in cats in Australia, New Zealand, and Canada.
References
- 1 2 3 4 5 6 Noble, S; Balfour, JA (March 1996). "Meloxicam". Drugs. 51 (3): 424–30, discussion 431–32. doi:10.2165/00003495-199651030-00007. PMID 8882380.
- 1 2 "Meloxicam official FDA information, side effects, and uses". Drugs.com. March 2010. Retrieved 17 March 2010.
- ↑ Auvinet, B; Ziller, R; Appelboom, T; Velicitat, P (November–December 1995). "Comparison of the onset and intensity of action of intramuscular meloxicam and oral meloxicam in patients with acute sciatica". Clinical Therapeutics. 17 (6): 1078–98. doi:10.1016/0149-2918(95)80086-7. PMID 8750399.
- ↑ Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 9. ISBN 9781284057560.
- ↑ Stamm O, Latscha U, Janecek P, et al. (January 1976). "Development of a special electrode for continuous subcutaneous pH measurement in the infant scalp". Am. J. Obstet. Gynecol. 124 (2): 193–5. PMID 2012.
- 1 2 Hawkey, C; Kahan, A; Steinbrück, K; Alegre, C; Baumelou, E; Bégaud, B; Dequeker, J; Isomäki, H; et al. (Sep 1998). "Gastrointestinal tolerability of meloxicam compared to diclofenac in osteoarthritis patients". Rheumatology. 37 (9): 937–945(9). doi:10.1093/rheumatology/37.9.937.
- ↑ Dequeker, J; Hawkey, C; Kahan, A; Steinbrück, K; Alegre, C; Baumelou, E; Begaud, B; Isomäki, H; et al. (1998). "Improvement in gastrointestinal tolerability of the selective cyclooxygenase (COX)-2 inhibitor, meloxicam, compared with piroxicam: results of the Safety and Efficacy Large-scale Evaluation of COX-inhibiting Therapies (SELECT) trial in osteoarthritis". The British Journal of Rheumatology. 37 (9): 946–51. doi:10.1093/rheumatology/37.9.946. PMID 9783758.
- ↑ Wojtulewski, JA; Schattenkirchner, M; Barceló, P; Le Loët, X; Bevis, PJR; Bluhmki, E; Distel, M. "A Six-Month Double-Blind Trial to Compare the Efficacy and Safety of Meloxicam 7.5 mg Daily and Naproxen 750 mg Daily in Patients with Rheumatoid Arthritis". Rheumatology. 35, Supplement 1: 22–8. doi:10.1093/rheumatology/35.suppl_1.22.
- ↑ Singh, G; Lanes, S; Triadafilopoulos, G (2004). "Gastrointestinal tolerability of meloxicam compared to diclofenac in osteoarthritis patients". Am J Med. 117 (9): 100–6. doi:10.1016/j.amjmed.2004.03.012. PMID 15234645.
- ↑ "Medline Plus". Nlm.nih.gov. Retrieved 15 November 2014.
- ↑ "Drugs.com". Drugs.com. Retrieved 15 November 2014.
- ↑ Engelhardt, G; Homma, D; Schlegel, K; Utzmann, R; Schnitzler, C (Oct 1995). "Anti-inflammatory, analgesic, antipyretic and related properties of meloxicam, a new non-steroidal anti-inflammatory agent with favourable gastrointestinal tolerance". Inflammation Research. 44 (10): 423–433. doi:10.1007/BF01757699. PMID 8564518.
- ↑ Off-label use discussed in: Arnold Plotnick MS, DVM, ACVIM, ABVP, Pain Management using Metacam Archived 2011-07-14 at the Wayback Machine., and Stein, Robert, Perioperative Pain Management Part IV, Looking Beyond Butorphanol, Sep 2006, Veterinary Anesthesia & Analgesia Support Group.
- ↑ For off-label use example in rabbits, see Krempels, Dana, Hind Limb Paresis and Paralysis in Rabbits, University of Miami Biology Department.
- ↑ Boström, IM; Nyman, G; Hoppe, A; Lord, P (January 2006). "Effects of meloxicam on renal function in dogs with hypotension during anaesthesia". Veterinary anaesthesia and analgesia. 33 (1): 62–9. doi:10.1111/j.1467-2995.2005.00208.x. PMID 16412133.
- ↑ Höglund, OV; Dyall, B; Gräsman, V; Edner, A; Olsson, U; Höglund, K (1 November 2017). "Effect of non-steroidal anti-inflammatory drugs on postoperative respiratory and heart rate in cats subjected to ovariohysterectomy". Journal of feline medicine and surgery: 1098612X17742290. doi:10.1177/1098612X17742290. PMID 29165006.
- ↑ "Toxicology Brief: The 10 most common toxicoses in cats". Veterinarymedicine.dvm360.com. 2006-06-01. Retrieved 2018-09-16.
- ↑ Merola, Valentina, DVM, DABT, and Dunayer Eric, MS, VMD, DABT, The 10 most common toxicoses in cats, Toxicology Brief, Veterinary Medicine, pp. 340–342, June, 2006.
- 1 2 3 Khan, SA; McLean, MK (March 2012). "Toxicology of frequently encountered nonsteroidal anti-inflammatory drugs in dogs and cats". The Veterinary clinics of North America. Small animal practice. 42 (2): 289–306, vi–vii. doi:10.1016/j.cvsm.2012.01.003. PMID 22381180.
- ↑ Kimble, B.; Black, L. A.; Li, K. M.; Valtchev, P.; Gilchrist, S.; Gillett, A.; Higgins, D. P.; Krockenberger, M. B.; Govendir, M. (2013). "Pharmacokinetics of meloxicam in koalas (Phascolarctos cinereus) after intravenous, subcutaneous and oral administration". Journal of Veterinary Pharmacology and Therapeutics. 36 (5): 486–493. doi:10.1111/jvp.12038. PMID 23406022.
- ↑ "NADA 141-213: New Animal Drug Application Approval (for Metacam (meloxicam) 0.5 mg/mL and 1.5 mg/mL Oral Suspension)" (PDF). US Food and Drug Administration. April 15, 2003. Retrieved 24 July 2010.
- ↑ Metacam Client Information Sheet, product description: "Non-steroidal anti-inflammatory drug for oral use in dogs only", and in the "What Is Metacam" section in bold-face type: "Do not use in cats.", January 2005.
- ↑ "Metacam 5 mg/mL Solution for Injection" (PDF). Fda.gov. Retrieved 15 November 2014.
- ↑ "Metacam 5 mg/mL Solution for Injection, Supplemental Approval" (PDF). Fda.gov. October 28, 2004. Retrieved 15 November 2014.
- ↑ See the manufacturer's FAQ on its website, and its clinical dosing instructions for cats. Archived 2008-09-06 at the Wayback Machine.
- ↑ US FDA Notice of Violation for off-label use promotion, April 2005.
- ↑ Maddison, JE; Page, SW; Church, D, eds. (2008). "Meloxicam". Small animal clinical pharmacology (2nd ed.). Edinburgh: Saunders/Elsevier. pp. 301–302. ISBN 9780702028588.