Medical uses of bicalutamide
The medical uses of bicalutamide, a nonsteroidal antiandrogen (NSAA), include the treatment of androgen-dependent conditions and hormone therapy to block the effects of androgens. Such indications include the treatment of prostate cancer in men, skin and hair conditions such as acne, seborrhea, hirsutism, and pattern hair loss in women, high testosterone levels in women, hormone therapy in transgender women, as a puberty blocker to prevent puberty in transgender girls and to treat early puberty in boys, and the treatment of long-lasting erections in men. It may also have some value in the treatment of paraphilias and hypersexuality in men.
Prostate cancer
Bicalutamide is used primarily in the treatment of early and advanced prostate cancer.[1] It is approved at a dosage of 50 mg/day as a combination therapy with a gonadotropin-releasing hormone analogue (GnRH analogue) or orchiectomy (that is, surgical or medical castration) in the treatment of stage D2 metastatic prostate cancer (mPC),[2][3] and as a monotherapy at a dosage of 150 mg/day for the treatment of stage C or D1 locally advanced prostate cancer (LAPC).[2][4][5][6] Although effective in mPC and LAPC, bicalutamide is no longer indicated for the treatment of localized prostate cancer (LPC) due to negative findings in the Early Prostate Cancer (EPC) trial.[5] Prior to the introduction of the newer NSAA enzalutamide in 2012,[7] bicalutamide was considered to be the standard-of-care antiandrogen in the treatment of prostate cancer, and still remains widely used for this indication.[8][7][9] Compared to earlier antiandrogens like the steroidal antiandrogen (SAA) cyproterone acetate (CPA) and the NSAAs flutamide and nilutamide, bicalutamide shows an improved profile of effectiveness, tolerability, and safety,[10][7][11][12] and for this reason has largely replaced them in the treatment of prostate cancer.[2][13][14][10][15][16][17]
In the early 1940s, it was discovered that growth of prostate cancer in men regressed with surgical castration or high-dose estrogen therapy, which produced very low levels of circulating testosterone, and accelerated with the administration of exogenous testosterone.[18][19] It has since been elucidated that androgens like testosterone and dihydrotestosterone (DHT) function as trophic factors for the prostate gland, stimulating cell division and proliferation and producing tissue growth and glandular enlargement, which, in the context of prostate cancer, results in stimulation of tumors and a considerable acceleration of disease progression.[20] As a result of these discoveries, androgen deprivation therapy (ADT), via a variety of modalities including surgical castration, high-dose estrogens, SAAs, GnRH analogues, NSAAs, and androgen biosynthesis inhibitors (e.g., abiraterone acetate), has become the mainstay of treatment for prostate cancer.[18] Although ADT can shrink or stabilize prostate tumors and hence significantly slow the course of prostate cancer and prolong life, it is, unfortunately, not generally curative.[21] While effective in slowing the progression of the disease initially, most advanced prostate cancer patients eventually become resistant to ADT and prostate cancer growth starts to accelerate again, in part due to progressive mutations in the androgen receptor (AR) that result in the transformation of drugs like bicalutamide from AR antagonists to agonists.[22]
A few observations form the basis of the reasoning behind combined androgen blockade (CAB), in which castration and an NSAA are combined.[23] It has been found that very low levels of androgens, as in castration, are able to significantly stimulate growth of prostate cancer cells and accelerate disease progression.[24] Although castration ceases production of androgens by the gonads and reduces circulating testosterone levels by about 95%,[25] low levels of androgens continue to be produced by the adrenal glands, and this accounts for the residual levels of circulating testosterone.[26] Moreover, it has been found that prostate gland levels of DHT, which is the major androgen in the prostate, remain at 40 to 50% of their initial values following castration.[26][27] This has been determined to be due to uptake of circulating weak adrenal androgens like dehydroepiandrosterone (DHEA) and androstenedione (A4) by the prostate and their de novo transformation into testosterone and DHT.[26][27][28] As such, a considerable amount of androgen signaling continues within the prostate gland even with castration.[26][27][28]
In the past, surgical adrenalectomy and early androgen biosynthesis inhibitors like ketoconazole and aminoglutethimide were successfully employed in the treatment of castration-resistant prostate cancer.[25][18][29][30] However, adrenalectomy is an invasive procedure with high morbidity, ketoconazole and aminoglutethimide have relatively high toxicity, and both treatment modalities require supplementation with corticosteroids, making them in many ways unideal.[18][31][32][33] The development of CAB with NSAAs like bicalutamide and enzalutamide and with newer and more tolerable androgen biosynthesis inhibitors like abiraterone acetate has since allowed for non-invasive, convenient, and well-tolerated therapies that have replaced the earlier modalities.[30][34]
Subsequent clinical research has found that monotherapy with higher dosages of NSAAs than those used in CAB is roughly equivalent to castration in extending life in men with prostate cancer.[10][18][35][36] Moreover, NSAA monotherapy is overall better tolerated and associated with greater quality of life than is castration,[37][38][39][40] which is thought to be related to the fact that testosterone levels do not decrease with NSAA monotherapy and hence by extension that levels of biologically active and beneficial metabolites of testosterone such as estrogens and neurosteroids are preserved.[39][40][41][42][43] For these reasons, NSAA monotherapy has become an important alternative to castration and CAB in the treatment of prostate cancer.[44][45][46]
Bicalutamide may be used to reduce the effects of the testosterone flare at the initiation of GnRH agonist therapy.[47][48]
Skin and hair conditions
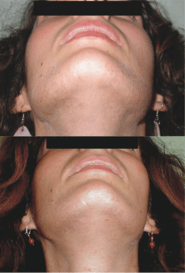
Androgens like testosterone and DHT play a critical role in the pathogenesis of a number of dermatological conditions including acne, seborrhea, hirsutism (excessive facial/body hair growth in women), and pattern hair loss (androgenic alopecia).[50] In demonstration of this, women with complete androgen insensitivity syndrome (CAIS) do not produce sebum or develop acne and have little to no body, pubic, or axillary hair.[51][52] Moreover, men with congenital 5α-reductase type II deficiency, 5α-reductase being an enzyme that greatly potentiates the androgenic effects of testosterone in the skin, have little to no acne, scanty facial hair, reduced body hair, and reportedly no incidence of male pattern hair loss.[53][54][55][56][49] Conversely, hyperandrogenism in women, for instance due to polycystic ovary syndrome (PCOS) or congenital adrenal hyperplasia (CAH), is commonly associated with acne and hirsutism as well as virilization (masculinization) in general.[50] In accordance with the preceding, antiandrogens have been found to be highly effective in the treatment of the aforementioned androgen-dependent skin and hair conditions.[57][58]
Low-dose bicalutamide has been found to be effective in the treatment of hirsutism in women in clinical studies.[59][60][61][62] In one of the studies, the drug was well-tolerated, all of the patients experienced a visible decrease in hair density, and a highly significant clinical improvement was observed with the Ferriman–Gallwey score decreasing by 41.2% at 3 months and by 61.6% at 6 months.[63][64] According to a 2013 review, "Low dose bicalutamide (25 mg/day) was shown to be effective in the treatment of hirsutism related to IH and PCOS. It does not have any significant side effects [or lead] to irregular periods."[60]
In addition to hirsutism, bicalutamide can also be used in the treatment of acne in women.[65][66] Several studies have observed complete clearing of acne with flutamide in women, and similar benefits would be expected with bicalutamide.[67]:712–717 Bicalutamide may also treat other androgen-dependent skin and hair conditions like seborrhea and pattern hair loss.[68] Flutamide has been found to produce a decrease of hirsutism score to normal and an 80% or greater decrease in scores of acne, seborrhea, and androgen-dependent hair loss.[69] Moreover, in combination with an oral contraceptive, flutamide treatment resulted in an increase in cosmetically acceptable scalp hair density in 6 of 7 women suffering from pattern hair loss.[67]
Antiandrogens like flutamide and bicalutamide are male-specific teratogens which can feminize male fetuses due to their antiandrogen effects,[57][70][71] and for this reason, are not recommended by the U.S. Food and Drug Administration for use in women.[72] Because of this risk, it is strongly recommended that antiandrogens only be used to treat women who are of reproductive age in conjunction with adequate contraception.[57][70][71] Oral contraceptives, which contain an estrogen and a progestin, are typically used for this purpose.[57] Moreover, oral contraceptives themselves are functional antiandrogens and are independently effective in the treatment of androgen-dependent skin and hair conditions, and hence can significantly augment the effectiveness of antiandrogens in the treatment of such conditions.[57][73]
Transgender hormone therapy
Bicalutamide is used as a component of feminizing hormone therapy for transgender women.[74][75][76][77][78][79] Beneficial or desired effects consist of demasculinization and feminization, and include breast development,[80][81][82] reduced male-pattern hair,[83] decreased muscle mass,[84] feminine changes in fat distribution, lowered libido,[84] and loss of spontaneous erections.[85] Unlike various other antiandrogens, bicalutamide significantly increases testosterone and estradiol levels in biological males when used as a monotherapy, and hence can have indirect estrogenic effects in transgender women.[86][87] This can be considered to be desirable in transgender women, as it can produce or contribute to feminization.[86][87][88] It should be noted that bicalutamide does not increase sex hormone levels if combined with an antigonadotropin such as a GnRH analogue, an estrogen, or a progestogen, due to negative feedback of these agents on sex hormone production.[47][89][90] Because bicalutamide does not lower androgen levels, it may be a favorable option for transgender women who wish to preserve sex drive and fertility.[91]
Unlike the SAAs CPA and spironolactone, as well as GnRH analogues,[92] few clinical studies assessing bicalutamide as an antiandrogen in transgender women have been conducted to date.[93][88] In any case, bicalutamide is clinically effective as an antiandrogen in women with hirsutism[59][60] and in boys with precocious puberty,[94][95][96][97] and demasculinization and feminization are well-documented effects of bicalutamide in men treated with it for prostate cancer.[83][98][99] In addition, nilutamide, a closely related antiandrogen that possesses the same mechanism of action as bicalutamide, has been evaluated in transgender women in at least five small clinical studies.[86][89][90][100][101][102] It was given at a high dosage of 300 mg/day, the same dosage in which it has been used as a monotherapy in the treatment of prostate cancer.[86][89][90][100][101][102] The corresponding monotherapy dosage of bicalutamide in the treatment of prostate cancer is 150 mg/day.[2][4][5]
In the clinical studies of nilutamide for transgender hormone therapy, the drug, without being combined with estrogen, induced observable signs of clinical feminization in young transgender women (age range 19–33 years) within 8 weeks,[90] including breast development, decreased male-pattern hair,[89] decreased spontaneous erections and sex drive,[100] and positive psychological and emotional changes.[100][103] Signs of breast development occurred in all subjects within 6 weeks and were associated with increased nipple sensitivity;[102][90][100] along with decreased hair growth, these changes were the earliest signs of feminization.[90] The drug more than doubled luteinizing hormone (LH) and testosterone levels and tripled estradiol levels,[89][90][101] and the addition of ethinylestradiol, a potent estrogen, to nilutamide therapy after 8 weeks of treatment abolished the increase in LH, testosterone, and estradiol levels and dramatically suppressed testosterone levels, into the castrate range.[89][90] On the basis of these results, both nilutamide alone, and particularly the combination of nilutamide and an estrogen, were regarded as effective in terms of producing antiandrogen effects and feminization in transgender women.[89][90]
Although nilutamide has been found to be effective for transgender hormone therapy, the use of nilutamide in the treatment of prostate cancer and particularly for other indications that are of a less dire nature is now discouraged due to the unique adverse effects of the medication, most importantly a high incidence of interstitial pneumonitis.[20][104][105] This is an adverse effect that can progress to pulmonary fibrosis and can potentially be fatal.[106] For this reason, newer, safer NSAAs like bicalutamide have largely replaced nilutamide and are now used for such indications instead.[15][16][17]
Madeline Deutsch of the Center of Excellence for Transgender Health at the University of California, San Francisco, in her Guidelines for the Primary and Gender-Affirming Care of Transgender and Gender Nonbinary People (2016), has said the following on the topic of bicalutamide in transgender women:[78]
In many countries, cyproterone acetate, a synthetic progestagen with strong anti-androgen activity is commonly used [as an antiandrogen in feminizing hormone therapy for transgender women]. Cyproterone [acetate] has been associated with uncommon episodes of fulminant hepatitis.[12] Bicalutamide, a direct anti-androgen used for the treatment of prostate cancer, also has a small but not fully quantified risk of liver function abnormalities (including several cases of fulminant hepatitis); while such risks are acceptable when considering the benefits of bicalutamide in the management of prostate cancer, such risks are less justified in the context of gender-affirming treatment.[13] No evidence at present exists to inform such an analysis.[78]
In spite of its risk of hepatotoxicity, CPA is the most widely used antiandrogen in Europe and elsewhere in the world for hormone therapy in transgender women.[86][107][108] In addition, it is widely used in cisgender women for the treatment of acne and hirsutism.[109] In the U.S., one of the only countries where CPA has not been approved for medical use,[110][111] the relatively weak agent spironolactone is the antiandrogen most commonly used in transgender and cisgender women instead.[112][113][114] Although both CPA and bicalutamide have been associated with hepatotoxicity (while spironolactone is not usually associated with such), bicalutamide has a far lower and only small risk of both elevated liver enzymes and hepatotoxicity;[115] whereas CPA has been associated with over 100 reports of hepatotoxicity,[116] five cases of hepatotoxicity, out of millions of patient exposures, have been associated with bicalutamide to date.[117][118]
In 2017, the first clinical study of bicalutamide in transgender females was published.[88]:477 The study specifically assessed bicalutamide as a puberty blocker to prevent puberty in adolescent transgender girls alone or in combination with an estrogen.[88] The subjects were 14 transgender girls with a mean age of 15.8 years (range 12 to 18.4 years) who were treated with 50 mg/day bicalutamide between 2013 and 2017, of whom seven returned for follow-up.[88] In addition to showing effectiveness as an antiandrogen in the subjects, bicalutamide monotherapy increased estradiol levels and promoted feminization as a secondary effect.[88] This included breast development to Tanner stage II and III in 86% of the subjects after an average of 5.7 months of treatment, with the seventh girl showing Tanner stage III breast development at her second follow-up at 12.5 months.[88] Testosterone levels were 524 to 619 ng/dL and estradiol levels were 26 to 61 pg/mL in the subjects.[88] Liver function tests were unremarkable.[88] Although GnRH analogues are the first-line treatment to prevent puberty in transgender adolescents, they are very expensive and are often denied by medical insurance.[88] According to the researchers, bicalutamide represents a potential alternative to GnRH analogues as a puberty blocker in transgender girls.[88] However, they expressed that additional studies are necessary to further evaluate the potential of bicalutamide for this use.[88]
Male early puberty
Bicalutamide (25–50 mg/day) is useful in combination with the aromatase inhibitor anastrozole as a puberty blocker in the treatment of male precocious puberty.[94][95][96] This is potentially a cost-effective alternative to GnRH analogues for the treatment of this condition, as GnRH analogues are very expensive.[119][120] Moreover, the combination is effective in gonadotropin-independent precocious puberty, namely familial male-limited precocious puberty (also known as testotoxicosis), where GnRH analogues notably are not effective.[95][96][121] Bicalutamide has been found to be superior to the SAA spironolactone (which has also been used, in combination with the aromatase inhibitor testolactone) for this indication; it has shown greater effectiveness and possesses fewer side effects in comparison.[95][122] For this reason, bicalutamide has replaced spironolactone in the treatment of the condition.[97]:2139
Long-lasting erections
Antiandrogens can considerably relieve and prevent priapism (potentially painful penile erections that last more than four hours) via direct blockade of penile ARs.[123][124] In accordance, bicalutamide, at low dosages (50 mg every other day or as little as once or twice weekly), has been found in a series of case reports to completely resolve recurrent priapism in men without producing significant side effects,[125][126][127] and is used for this indication off-label.[128][129] In the reported cases, libido, rigid erections, the potential for sexual intercourse, orgasm, and subjective ejaculatory volume have all remained intact or unchanged, and gynecomastia has not developed when bicalutamide is administered at a total dosage of 25 mg/day or less.[125][126][127] Some gynecomastia and breast tenderness developed in one patient treated with 50 mg/day, but significantly improved upon the dosage being halved.[127] The observed tolerability profile of bicalutamide in these subjects has been regarded as significantly more favorable than that of GnRH analogues and estrogens (which are also used in the treatment of this condition).[125][126] However, although successful and well-tolerated, very few cases have been reported.[130]
Paraphilias and hypersexuality
The antigonadotropic antiandrogens CPA, medroxyprogesterone acetate (MPA), and GnRH analogues have all been widely used to treat paraphilias (e.g., pedophilia) and hypersexuality in men.[131][132] They suppress androgen levels to castrate or near-castrate levels and are highly effective in reducing sexual urges, arousal, and behaviors.[131] In addition, they are used to treat sex offenders as a means of chemical castration for the purpose of reducing the likelihood of recidivism.[131]
Although they have not been studied in the treatment of paraphilias and hypersexuality, NSAAs like flutamide and bicalutamide have been suggested as potential medications for these indications and may have superior tolerability and safety relative to antigonadotropic antiandrogens.[131][133][134][135] As an example, because NSAAs do not reduce estrogen levels, unlike antigonadotropic antiandrogens, they preserve bone mineral density (BMD) and have little or no risk of osteoporosis and associated bone fractures.[131][133][136] However, due to unopposed estrogen signaling, a substantial incidence of gynecomastia is associated with NSAAs.[131] In addition to potential monotherapy use, NSAAs have been advocated for temporarily suppressing sex drive during the start of GnRH agonist treatment via prevention of the increased androgen signaling associated with the initial testosterone flare.[132]
Though treatment of paraphilias and hypersexuality with selective AR antagonists is a seemingly sound strategy, this may not be true in practice.[38] Surprisingly, little or no sexual dysfunction, including loss of sex drive and decreased sexual activity, has been observed in clinical studies of NSAA monotherapy.[38][5] The explanation for this is that NSAAs do not lower androgen levels, and metabolites of testosterone like estrogens and neurosteroids may be of critical importance for maintenance of sex drive and function in males.[39][40][41][42][43] In accordance, testosterone is locally aromatized into estradiol widely throughout the brain and estradiol appears to be the mediator of many of the central actions of testosterone.[57] For these reasons, unlike antigonadotropic antiandrogens, NSAA monotherapy may have limited usefulness in the management of paraphilias and hypersexuality.[38] The addition of NSAAs to antigonadotropic antiandrogens like GnRH analogues may have some usefulness however, particularly in severe cases.[132][137] In any case, insufficient evidence is available at this time, and further research is thus warranted.[133]
References
- ↑ Bagatelle C, Bremner WJ (27 May 2003). Androgens in Health and Disease. Springer Science & Business Media. pp. 25–. ISBN 978-1-59259-388-0.
- 1 2 3 4 Lemke TL, Williams DA (2008). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 121, 1288, 1290. ISBN 978-0-7817-6879-5. Archived from the original on 8 September 2017.
- ↑ Klotz L, Schellhammer P (March 2005). "Combined androgen blockade: the case for bicalutamide". Clinical Prostate Cancer. 3 (4): 215–9. doi:10.3816/cgc.2005.n.002. PMID 15882477.
- 1 2 Cockshott ID (2004). "Bicalutamide: clinical pharmacokinetics and metabolism". Clinical Pharmacokinetics. 43 (13): 855–878. doi:10.2165/00003088-200443130-00003. PMID 15509184.
These data indicate that direct glucuronidation is the main metabolic pathway for the rapidly cleared (S)-bicalutamide, whereas hydroxylation followed by glucuronidation is a major metabolic pathway for the slowly cleared (R)-bicalutamide.
- 1 2 3 4 Wellington K, Keam SJ (2006). "Bicalutamide 150mg: a review of its use in the treatment of locally advanced prostate cancer" (PDF). Drugs. 66 (6): 837–50. doi:10.2165/00003495-200666060-00007. PMID 16706554. Archived (PDF) from the original on 28 August 2016.
- ↑ Schellhammer PF, Sharifi R, Block NL, Soloway MS, Venner PM, Patterson AL, Sarosdy MF, Vogelzang NJ, Schellenger JJ, Kolvenbag GJ (September 1997). "Clinical benefits of bicalutamide compared with flutamide in combined androgen blockade for patients with advanced prostatic carcinoma: final report of a double-blind, randomized, multicenter trial. Casodex Combination Study Group". Urology. 50 (3): 330–6. doi:10.1016/S0090-4295(97)00279-3. PMID 9301693.
- 1 2 3 Vogelzang NJ (September 2012). "Enzalutamide—a major advance in the treatment of metastatic prostate cancer". The New England Journal of Medicine. 367 (13): 1256–7. doi:10.1056/NEJMe1209041. PMID 23013078.
The first nonsteroidal antiandrogen agents — flutamide, nilutamide, and bicalutamide2 — were shown to be less effective than castration in cases of metastatic castration-resistant prostate cancer, but bicalutamide is still widely used as a moderately effective secondary hormone therapy because of an excellent safety profile.
- ↑ Regitz-Zagrosek V (2 October 2012). Sex and Gender Differences in Pharmacology. Springer Science & Business Media. pp. 575–. ISBN 978-3-642-30725-6. Archived from the original on 24 June 2016.
- ↑ Horwich A (11 February 2010). Systemic Treatment of Prostate Cancer. OUP Oxford. pp. 44–. ISBN 978-0-19-956142-1.
- 1 2 3 Chabner BA, Longo DL (8 November 2010). Cancer Chemotherapy and Biotherapy: Principles and Practice. Lippincott Williams & Wilkins. pp. 679–680. ISBN 978-1-60547-431-1.
From a structural standpoint, antiandrogens are classified as steroidal, including cyproterone [acetate] (Androcur) and megestrol [acetate], or nonsteroidal, including flutamide (Eulexin, others), bicalutamide (Casodex), and nilutamide (Nilandron). The steroidal antiandrogens are rarely used.
- ↑ Weber GF (22 July 2015). Molecular Therapies of Cancer. Springer. pp. 318–. ISBN 978-3-319-13278-5.
Compared to flutamide and nilutamide, bicalutamide has a 2-fold increased affinity for the Androgen Receptor, a longer half-life, and substantially reduced toxicities. Based on a more favorable safety profile relative to flutamide, bicalutamide is indicated for use in combination therapy with a Gonadotropin Releasing Hormone analog for the treatment of advanced metastatic prostate carcinoma.
- ↑ Kolvenbag GJ, Blackledge GR (January 1996). "Worldwide activity and safety of bicalutamide: a summary review". Urology. 47 (1A Suppl): 70–9, discussion 80–4. doi:10.1016/s0090-4295(96)80012-4. PMID 8560681.
Bicalutamide is a new antiandrogen that offers the convenience of once-daily administration, demonstrated activity in prostate cancer, and an excellent safety profile. Because it is effective and offers better tolerability than flutamide, bicalutamide represents a valid first choice for antiandrogen therapy in combination with castration for the treatment of patients with advanced prostate cancer.
- ↑ Kaliks RA, Del Giglio A (2008). "Management of advanced prostate cancer" (PDF). Revista Da Associação Médica Brasileira. 54 (2): 178–82. doi:10.1590/S0104-42302008000200025. PMID 18506331. Archived (PDF) from the original on 10 May 2017.
- ↑ Payen O, Top S, Vessières A, Brulé E, Lauzier A, Plamont M, McGlinchey MJ, Müller-Bunz H, Jaouen G (2011). "Synthesis and biological activity of ferrocenyl derivatives of the non-steroidal antiandrogens flutamide and bicalutamide" (PDF). Journal of Organometallic Chemistry. 696 (5): 1049–1056. doi:10.1016/j.jorganchem.2010.10.051.
Cyproterone acetate was one of the first steroidal antiandrogen clinically used but its side-effects, especially the interaction with the progestin and glucocorticoid receptor, made this drug less popular than the nonsteroidal antiandrogens such as nilutamide [3,4], flutamide [5–7] and bicalutamide [8].
- 1 2 Gulley JL (2011). Prostate Cancer. Demos Medical Publishing. pp. 81–. ISBN 978-1-935281-91-7. Archived from the original on 25 April 2016.
- 1 2 Moser L (1 January 2008). Controversies in the Treatment of Prostate Cancer. Karger Medical and Scientific Publishers. pp. 41–42. ISBN 978-3-8055-8524-8. Archived from the original on 16 May 2016.
- 1 2 Gulley JL (20 December 2011). Prostate Cancer. Demos Medical Publishing. pp. 505–. ISBN 978-1-935281-91-7.
- 1 2 3 4 5 Figg W, Chau CH, Small EJ (14 September 2010). Drug Management of Prostate Cancer. Springer Science & Business Media. pp. 56, 71–72, 75, 93. ISBN 978-1-60327-829-4.
- ↑ Kavoussi P, Costabile RA, Salonia A (17 October 2012). Clinical Urologic Endocrinology: Principles for Men’s Health. Springer Science & Business Media. pp. 7–. ISBN 978-1-4471-4404-5.
- 1 2 Denis LJ, Griffiths K, Kaisary AV, Murphy GP (1 March 1999). Textbook of Prostate Cancer: Pathology, Diagnosis and Treatment: Pathology, Diagnosis and Treatment. CRC Press. pp. 55, 279–280. ISBN 978-1-85317-422-3. Archived from the original on 3 June 2016.
- ↑ Munoz J, Wheler JJ, Kurzrock R (2015). "Androgen receptors beyond prostate cancer: an old marker as a new target". Oncotarget. 6 (2): 592–603. doi:10.18632/oncotarget.2831. PMC 4359241. PMID 25595907.
- ↑ Balk SP (September 2002). "Androgen receptor as a target in androgen-independent prostate cancer". Urology. 60 (3 Suppl 1): 132–8, discussion 138–9. doi:10.1016/S0090-4295(02)01593-5. PMID 12231070.
- ↑ Sarosdy MF (1999). "Which is the optimal antiandrogen for use in combined androgen blockade of advanced prostate cancer? The transition from a first- to second-generation antiandrogen". Anticancer Drugs. 10 (9): 791–6. doi:10.1097/00001813-199910000-00001. PMID 10587288.
- ↑ Dasgupta P, Kirby RS (27 December 2011). ABC of Prostate Cancer. John Wiley & Sons. pp. 52–. ISBN 978-1-4443-3437-1.
- 1 2 Bruskewitz R (6 December 2012). Atlas of the Prostate. Springer Science & Business Media. pp. 5, 190. ISBN 978-1-4615-6505-5.
- 1 2 3 4 Denis L (6 December 2012). Antiandrogens in Prostate Cancer: A Key to Tailored Endocrine Treatment. Springer Science & Business Media. pp. 128, 158, 203. ISBN 978-3-642-45745-6.
- 1 2 3 Luo S, Martel C, Chen C, Labrie C, Candas B, Singh SM, Labrie F (December 1997). "Daily dosing with flutamide or Casodex exerts maximal antiandrogenic activity". Urology. 50 (6): 913–9. doi:10.1016/S0090-4295(97)00393-2. PMID 9426723.
- 1 2 Gauthier S, Martel C, Labrie F (October 2012). "Steroid derivatives as pure antagonists of the androgen receptor". The Journal of Steroid Biochemistry and Molecular Biology. 132 (1–2): 93–104. doi:10.1016/j.jsbmb.2012.02.006. PMID 22449547.
- ↑ Lupulescu A (24 October 1990). Hormones and Vitamins in Cancer Treatment. CRC Press. pp. 113–. ISBN 978-0-8493-5973-6.
- 1 2 Petrovich Z, Baert L, Brady LW (6 December 2012). Carcinoma of the Prostate: Innovations in Management. Springer Science & Business Media. pp. 687–. ISBN 978-3-642-60956-5.
- ↑ Held-Warmkessel J (2006). Contemporary Issues in Prostate Cancer: A Nursing Perspective. Jones & Bartlett Learning. pp. 275–. ISBN 978-0-7637-3075-8.
- ↑ Steckler T, Kalin N, Reul J (25 February 2005). Handbook of Stress and the Brain Part 2: Stress: Integrative and Clinical Aspects. Elsevier. pp. 442–. ISBN 978-0-08-055331-3.
- ↑ Hong WK, Holland JF (2010). Holland-Frei Cancer Medicine 8. PMPH-USA. pp. 939–. ISBN 978-1-60795-014-1.
- ↑ Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA (25 August 2011). Campbell-Walsh Urology: Expert Consult Premium Edition: Enhanced Online Features and Print, 4-Volume Set. Elsevier Health Sciences. pp. 2938–2939, 2946. ISBN 978-1-4160-6911-9. Archived from the original on 5 May 2016.
- ↑ Mydlo JH, Godec CJ (29 September 2015). Prostate Cancer: Science and Clinical Practice. Elsevier Science. pp. 516–521, 534–540. ISBN 978-0-12-800592-7. Archived from the original on 8 September 2017.
- ↑ Strauss III JF, Barbieri RL (28 August 2013). Yen & Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. Elsevier Health Sciences. pp. 688–. ISBN 978-1-4557-5972-9.
Bone density improves in men receiving bicalutamide, most likely secondary to the 146% increase in estradiol and the fact that estradiol is the major mediator of bone density in men.
- ↑ Fradet Y (February 2004). "Bicalutamide (Casodex) in the treatment of prostate cancer". Expert Review of Anticancer Therapy. 4 (1): 37–48. doi:10.1586/14737140.4.1.37. PMID 14748655.
In contrast, the incidence of diarrhea was comparable between the bicalutamide and placebo groups (6.3 vs. 6.4%, respectively) in the EPC program [71].
- 1 2 3 4 Iversen P, Melezinek I, Schmidt A (January 2001). "Nonsteroidal antiandrogens: a therapeutic option for patients with advanced prostate cancer who wish to retain sexual interest and function". BJU International. 87 (1): 47–56. doi:10.1046/j.1464-410x.2001.00988.x. PMID 11121992.
- 1 2 3 Wibowo E, Schellhammer P, Wassersug RJ (January 2011). "Role of estrogen in normal male function: clinical implications for patients with prostate cancer on androgen deprivation therapy". The Journal of Urology. 185 (1): 17–23. doi:10.1016/j.juro.2010.08.094. PMID 21074215.
- 1 2 3 Motofei IG, Rowland DL, Popa F, Kreienkamp D, Paunica S (July 2011). "Preliminary study with bicalutamide in heterosexual and homosexual patients with prostate cancer: a possible implication of androgens in male homosexual arousal". BJU International. 108 (1): 110–5. doi:10.1111/j.1464-410X.2010.09764.x. PMID 20955264.
- 1 2 Wibowo E, Wassersug RJ (September 2013). "The effect of estrogen on the sexual interest of castrated males: Implications to prostate cancer patients on androgen-deprivation therapy". Critical Reviews in Oncology/Hematology. 87 (3): 224–38. doi:10.1016/j.critrevonc.2013.01.006. PMID 23484454.
- 1 2 Chedrese PJ (13 June 2009). Reproductive Endocrinology: A Molecular Approach. Springer Science & Business Media. pp. 233–. ISBN 978-0-387-88186-7. Archived from the original on 5 September 2017.
- 1 2 King SR (2008). "Emerging roles for neurosteroids in sexual behavior and function". Journal of Andrology. 29 (5): 524–33. doi:10.2164/jandrol.108.005660. PMID 18567641.
- ↑ Anderson J (March 2003). "The role of antiandrogen monotherapy in the treatment of prostate cancer". BJU International. 91 (5): 455–61. doi:10.1046/j.1464-410X.2003.04026.x. PMID 12603397.
- ↑ Boccardo F (August 2000). "Hormone therapy of prostate cancer: is there a role for antiandrogen monotherapy?". Critical Reviews in Oncology/Hematology. 35 (2): 121–32. doi:10.1016/S1040-8428(00)00051-2. PMID 10936469.
- ↑ Kolvenbag GJ, Iversen P, Newling DW (2001). "Antiandrogen monotherapy: a new form of treatment for patients with prostate cancer". Urology. 58 (2 Suppl 1): 16–23. doi:10.1016/s0090-4295(01)01237-7. PMID 11502439.
- 1 2 Shlomo Melmed (1 January 2016). Williams Textbook of Endocrinology. Elsevier Health Sciences. pp. 752–. ISBN 978-0-323-29738-7.
GnRH analogues, both agonists and antagonists, severely suppress endogenous gonadotropin and testosterone production [...] Administration of GnRH agonists (e.g., leuprolide, goserelin) produces an initial stimulation of gonadotropin and testosterone secretion (known as a "flare"), which is followed in 1 to 2 weeks by GnRH receptor downregulation and marked suppression of gonadotropins and testosterone to castration levels. [...] To prevent the potential complications associated with the testosterone flare, AR antagonists (e.g., bicalutamide) are usually coadministered with a GnRH agonist for men with metastatic prostate cancer.399
- ↑ Sugiono M, Winkler MH, Okeke AA, Benney M, Gillatt DA (2005). "Bicalutamide vs cyproterone acetate in preventing flare with LHRH analogue therapy for prostate cancer—a pilot study". Prostate Cancer and Prostatic Diseases. 8 (1): 91–4. doi:10.1038/sj.pcan.4500784. PMID 15711607.
- 1 2 Blume-Peytavi U, Whiting DA, Trüeb RM (26 June 2008). Hair Growth and Disorders. Springer Science & Business Media. pp. 161–162, 181. ISBN 978-3-540-46911-7.
- 1 2 Zouboulis CC, Degitz K (2004). "Androgen action on human skin -- from basic research to clinical significance". Experimental Dermatology. 13 Suppl 4: 5–10. doi:10.1111/j.1600-0625.2004.00255.x. PMID 15507105.
- ↑ Shalita AR, Del Rosso JQ, Webster G (21 March 2011). Acne Vulgaris. CRC Press. pp. 33–. ISBN 978-1-61631-009-7. Archived from the original on 9 December 2016.
- ↑ Zouboulis CC, Katsambas AD, Kligman AM (28 July 2014). Pathogenesis and Treatment of Acne and Rosacea. Springer. pp. 121–. ISBN 978-3-540-69375-8. Archived from the original on 10 December 2016.
- ↑ Marks LS (2004). "5alpha-reductase: history and clinical importance". Reviews in Urology. 6 Suppl 9: S11–21. PMC 1472916. PMID 16985920.
- ↑ Sloane E (2002). Biology of Women. Cengage Learning. pp. 160–. ISBN 0-7668-1142-5.
- ↑ Hanno PM, Guzzo TJ, Malkowicz SB, Wein AJ (26 January 2014). Penn Clinical Manual of Urology E-Book: Expert Consult - Online. Elsevier Health Sciences. pp. 782–. ISBN 978-0-323-24466-4.
- ↑ Harper C (1 August 2007). Intersex. Berg. pp. 123–. ISBN 978-1-84788-339-1.
- 1 2 3 4 5 6 Diamanti-Kandarakis E, Tolis G, Duleba AJ (1995). "Androgens and therapeutic aspects of antiandrogens in women". Journal of the Society for Gynocologic Investigation. 2 (4): 577–92. doi:10.1177/107155769500200401. PMID 9420861.
Androgen receptors have been detected in many areas including the cortex, pituitary, hypothalamus, preoptic area, septum, amygdala, thalamus, and brain stem.79,80 These receptors appear to be identical to other ARs and may be activated by both testosterone and 5α-DHT. In the cortex and pituitary, androgens do not undergo significant aromatization, and it appears that the effects of androgens on these tissues are mediated by ARs. In contrast, most of the effects of androgens on the hypothalamus and limbic system are thought to be mediated by estrogen receptors rather than AR.78 This concept, the aromatization hypothesis, has been validated in studies of the brain of the female subhuman primate.
- ↑ Katsambas AD, Dessinioti C (2010). "Hormonal therapy for acne: why not as first line therapy? facts and controversies". Clinics in Dermatology. 28 (1): 17–23. doi:10.1016/j.clindermatol.2009.03.006. PMID 20082945.
- 1 2 Williams H, Bigby M, Diepgen T, Herxheimer A, Naldi L, Rzany B (22 January 2009). Evidence-Based Dermatology. John Wiley & Sons. pp. 529–. ISBN 978-1-4443-0017-8. Archived from the original on 2 May 2016.
- 1 2 3 Erem C (2013). "Update on idiopathic hirsutism: diagnosis and treatment". Acta Clinica Belgica. 68 (4): 268–74. doi:10.2143/ACB.3267. PMID 24455796.
- ↑ Moretti C, Guccione L, Di Giacinto P, Simonelli I, Exacoustos C, Toscano V, Motta C, De Leo V, Petraglia F, Lenzi A (March 2018). "Combined Oral Contraception and Bicalutamide in Polycystic Ovary Syndrome and Severe Hirsutism: A Double-Blind Randomized Controlled Trial". J. Clin. Endocrinol. Metab. 103 (3): 824–838. doi:10.1210/jc.2017-01186. PMID 29211888.
- ↑ Costanzo Giulio Moretti, Laura Guccione, Paola Di Giacinto, Amalia Cannuccia, Chiara Meleca, Giulia Lanzolla, Aikaterini Andreadi, Davide Lauro (2016), Efficacy and Safety of Myo-Inositol Supplementation in the Treatment of Obese Hirsute PCOS Women: Comparative Evaluation with OCP+Bicalutamide Therapy, doi:10.1210/endo-meetings.2016.RE.5.SUN-153
- ↑ Bigby M, Herxheimer A, Naldi L, et al. (5 June 2014). Evidence-Based Dermatology. Wiley. pp. 1904–. ISBN 978-1-118-35762-0.
- ↑ Shapiro J (12 November 2012). Hair Disorders: Current Concepts in Pathophysiology, Diagnosis and Management, An Issue of Dermatologic Clinics. Elsevier Health Sciences. pp. 187–. ISBN 1-4557-7169-4.
- ↑ Ascenso A, Marques HC (January 2009). "Acne in the adult". Mini Reviews in Medicinal Chemistry. 9 (1): 1–10. doi:10.2174/138955709787001730. PMID 19149656.
- ↑ Kaur S, Verma P, Sangwan A, Dayal S, Jain VK (2016). "Etiopathogenesis and Therapeutic Approach to Adult Onset Acne". Indian Journal of Dermatology. 61 (4): 403–7. doi:10.4103/0019-5154.185703. PMC 4966398. PMID 27512185.
- 1 2 Diamanti-Kandarakis E (September 1999). "Current aspects of antiandrogen therapy in women". Current Pharmaceutical Design. 5 (9): 707–23. PMID 10495361.
Several trials demonstrated complete clearing of acne with flutamide [62,77]. Flutamide used in combination with an [oral contraceptive], at a dose of 500mg/d, flutamide caused a dramatic decrease (80%) in total acne, seborrhea and hair loss score after only 3 months of therapy [53]. When used as a monotherapy in lean and obese PCOS, it significantly improves the signs of hyperandrogenism, hirsutism and particularly acne [48]. [...] flutamide 500mg/d combined with an [oral contraceptive] caused an increase in cosmetically acceptable hair density, in sex of seven women suffering from diffuse androgenetic alopecia [53].
- ↑ Lotti F, Maggi M (2015). "Hormonal Treatment for Skin Androgen-Related Disorders". European Handbook of Dermatological Treatments: 1451–1464. doi:10.1007/978-3-662-45139-7_142.
- ↑ Singh SM, Gauthier S, Labrie F (February 2000). "Androgen receptor antagonists (antiandrogens): structure-activity relationships". Current Medicinal Chemistry. 7 (2): 211–47. doi:10.2174/0929867003375371. PMID 10637363.
- 1 2 Iswaran TJ, Imai M, Betton GR, Siddall RA (May 1997). "An overview of animal toxicology studies with bicalutamide (ICI 176,334)". The Journal of Toxicological Sciences. 22 (2): 75–88. doi:10.2131/jts.22.2_75. PMID 9198005.
- 1 2 Smith RE (4 April 2013). Medicinal Chemistry – Fusion of Traditional and Western Medicine. Bentham Science Publishers. pp. 306–. ISBN 978-1-60805-149-6. Archived from the original on 29 May 2016.
- ↑ "Bicalutamide". The American Society of Health-System Pharmacists. Archived from the original on 29 December 2016. Retrieved 8 December 2016.
- ↑ Adam Ostrzenski (2002). Gynecology: Integrating Conventional, Complementary, and Natural Alternative Therapy. Lippincott Williams & Wilkins. pp. 86–. ISBN 978-0-7817-2761-7.
- ↑ Gooren, LJ (31 March 2011). "Clinical practice. Care of transsexual persons". The New England Journal of Medicine. 364 (13): 1251–7. doi:10.1056/nejmcp1008161. PMID 21449788.
- ↑ Bourgeois AL, Auriche P, Palmaro A, Montastruc JL, Bagheri H (February 2016). "Risk of hormonotherapy in transgender people: Literature review and data from the French Database of Pharmacovigilance". Annales d'Endocrinologie. 77 (1): 14–21. doi:10.1016/j.ando.2015.12.001. PMID 26830952.
Drugs for cross-gender hormonal replacement therapy used in the male to female (MtoF) transsexual population. [...] Non-steroidal anti-androgens Bicalutamide, flutamide, nilutamide
- ↑ Ho CK (December 2011). "Testosterone testing in adult males". The Malaysian Journal of Pathology. 33 (2): 71–81. PMID 22299206.
Anti-androgens such as flutamide, bicalutamide and cyproterone acetate are also used in patients with prostate cancer and sometimes in male-to-female transgender individuals [...]
- ↑ Wierckx K, Gooren L, T'Sjoen G (May 2014). "Clinical review: Breast development in trans women receiving cross-sex hormones". The Journal of Sexual Medicine. 11 (5): 1240–7. doi:10.1111/jsm.12487. PMID 24618412.
Other agents with anti-androgenic properties used [in the treatment of transgender women] are nonsteroidal androgen receptor blockers, such as flutamide and bicalutamide [...]
- 1 2 3 Deutsch M (17 June 2016), Guidelines for the Primary and Gender-Affirming Care of Transgender and Gender Nonbinary People (PDF) (2nd ed.), University of California, San Francisco: Center of Excellence for Transgender Health, p. 28
- ↑ Benjamin Vincent (21 June 2018). Transgender Health: A Practitioner's Guide to Binary and Non-Binary Trans Patient Care. Jessica Kingsley Publishers. pp. 158–. ISBN 978-1-78450-475-5.
- ↑ Braunstein GD (September 2007). "Clinical practice. Gynecomastia". The New England Journal of Medicine. 357 (12): 1229–37. doi:10.1056/NEJMcp070677. PMID 17881754.
- ↑ Gentilini OD, Boccardo C (2015). "Male Breast Diseases". The Outpatient Breast Clinic: 431–446. doi:10.1007/978-3-319-15907-2_19.
- ↑ Kanhai RC, Hage JJ, van Diest PJ, Bloemena E, Mulder JW (January 2000). "Short-term and long-term histologic effects of castration and estrogen treatment on breast tissue of 14 male-to-female transsexuals in comparison with two chemically castrated men". The American Journal of Surgical Pathology. 24 (1): 74–80. doi:10.1097/00000478-200001000-00009. PMID 10632490.
- 1 2 Higano CS (February 2003). "Side effects of androgen deprivation therapy: monitoring and minimizing toxicity". Urology. 61 (2 Suppl 1): 32–8. doi:10.1016/S0090-4295(02)02397-X. PMID 12667885.
- 1 2 Lehne RA (2013). Pharmacology for Nursing Care. Elsevier Health Sciences. pp. 1297–. ISBN 1-4377-3582-7.
- ↑ el-Rayes BF, Hussain MH (February 2002). "Hormonal therapy for prostate cancer: past, present and future". Expert Review of Anticancer Therapy. 2 (1): 37–47. doi:10.1586/14737140.2.1.37. PMID 12113064.
At 2-year follow-up, loss of spontaneous erections and sexual function occurred in 80 vs. 92% and 78 vs. 88% in the flutamide versus CPA groups, respectively. This group of agents includes flutamide, nilutamide and bicalutamide.
- 1 2 3 4 5 Kreukels BP, Steensma TD, de Vries AL (1 July 2013). Gender Dysphoria and Disorders of Sex Development: Progress in Care and Knowledge. Springer Science & Business Media. pp. 280–. ISBN 978-1-4614-7441-8.
Nonsteroidal antiandrogens, such as flutamide (50–75 mg/day) and nilutamide (150 mg/day), are also used, but they increase gonadotropin output with a rise of testosterone and estradiol; the rise of estradiol is a desirable effect in this context.
- 1 2 Jameson JL, de Kretser DM, Marshall JC, De Groot LJ (7 May 2013). Endocrinology Adult and Pediatric: Reproductive Endocrinology. Elsevier Health Sciences. ISBN 978-0-323-22152-8. Archived from the original on 25 July 2014.
Nonsteroidal antiandrogens (e.g., flutamide and nilutamide) are also used, but they increase gonadotropin secretion, causing increased secretion of testosterone and estradiol.119 The latter is desirable in this context, as it has feminizing effects.
- 1 2 3 4 5 6 7 8 9 10 11 12 Neyman, A; Fuqua, JS; Eugster, EA (December 2017). "Bicalutamide as an Androgen Blocker with Secondary Effect of Promoting Feminization in Male to Female (MTF) Transgender Adolescents". Hormone Research in Paediatrics. 88: 1–628. doi:10.1159/000481424. PMID 28968603.
Abstract. Objectives: GnRH analogs are first-line treatment for halting pubertal development in gender variant youth. However, this medication is often denied by third party payors. The pure androgen receptor blocker bicalutamide represents a potential alternative approach to blocking puberty in natal males. Here, we describe the use of bicalutamide in MTF transgender patients. Methods: Medical records for patients with gender dysphoria (GD) followed in the pediatric endocrine clinic at Riley Hospital for Children were reviewed. All MTF transgender patients treated with bicalutamide were included. Variables evaluated comprised age, duration of follow up, timing of estrogen initiation, laboratory studies and physical exam findings including change in breast Tanner stage during treatment. Results: Of 77 patients with GD identified, 29 were MTF, of whom 14 (48%) aged 15.8 ± 1.9 years (range 12-18.4yr) were treated with bicalutamide 50 mg daily between 2013 and 2017. Of these, 3 were started on estrogen concurrently whereas 11 received bicalutamide alone, 7 of whom have returned for follow up thus far. After an average of 5.7±1.5 months, 86% of the patients (n=6) had breast development consisting of Tanner stage III in 4, Tanner stage II in 1, and Tanner stage III/11 of the right and left breast in 1. The 7th patient was noted to have Tanner Ill breasts at her 2nd follow-up clinic visit 12.5 months after starting bicalutamide. LFTs were obtained on 4 patients, estradiol on 3 patients and testosterone on 2 patients while exclusively taking bicalutamide. LFTs were unremarkable and concentrations of estradiol and testosterone were 26-61 pg/mL and 524-619 ng/dL, respectively. Conclusions: Bicalutamide is used in rare forms of precocious puberty in males and has a known side effect of gynecomastia. Here, we report the novel use of bicalutamide as a puberty blocker in MTF patients with GD in whom it also results in feminization by causing breast development. Additional studies are needed to further evaluate the potential role of bicalutamide in the therapeutic armamentarium for the treatment of transgender MTF adolescents.
- 1 2 3 4 5 6 7 Asscheman H, Gooren LJ, Peereboom-Wynia JD (1989). "Reduction in undesired sexual hair growth with anandron in male-to-female transsexuals—experiences with a novel androgen receptor blocker". Clinical and Experimental Dermatology. 14 (5): 361–3. doi:10.1111/j.1365-2230.1989.tb02585.x. PMID 2612040.
- 1 2 3 4 5 6 7 8 9 Rao BR, de Voogt HJ, Geldof AA, Gooren LJ, Bouman FG (1988). "Merits and considerations in the use of anti-androgen". Journal of Steroid Biochemistry. 31 (4B): 731–7. doi:10.1016/0022-4731(88)90024-6. PMID 3143862.
- ↑ Gao Y, Maurer T, Mirmirani P (January 2018). "Understanding and Addressing Hair Disorders in Transgender Individuals". Am J Clin Dermatol. doi:10.1007/s40257-018-0343-z. PMID 29352423.
Non-steroidal antiandrogens include flutamide, nilutamide, and bicalutamide, which do not lower androgen levels and may be favorable for individuals who want to preserve sex drive and fertility [9].
- ↑ Moore E, Wisniewski A, Dobs A (2003). "Endocrine treatment of transsexual people: a review of treatment regimens, outcomes, and adverse effects". The Journal of Clinical Endocrinology & Metabolism. 88 (8): 3467–73. doi:10.1210/jc.2002-021967. PMID 12915619.
- ↑ ""bicalutamide" OR "casodex" "transgender" OR "transsexual" OR "transsexuals" OR "trans women" OR "male-to-female" - Google Scholar". Retrieved 2017-12-25.
- 1 2 Kliegman RM, Stanton B, St Geme J, Schor NF (17 April 2015). Nelson Textbook of Pediatrics. Elsevier Health Sciences. pp. 2661–. ISBN 978-0-323-26352-8.
- 1 2 3 4 Kreher NC, Pescovitz OH, Delameter P, Tiulpakov A, Hochberg Z (September 2006). "Treatment of familial male-limited precocious puberty with bicalutamide and anastrozole". The Journal of Pediatrics. 149 (3): 416–20. doi:10.1016/j.jpeds.2006.04.027. PMID 16939760.
- 1 2 3 Reiter EO, Mauras N, McCormick K, Kulshreshtha B, Amrhein J, De Luca F, O'Brien S, Armstrong J, Melezinkova H (October 2010). "Bicalutamide plus anastrozole for the treatment of gonadotropin-independent precocious puberty in boys with testotoxicosis: a phase II, open-label pilot study (BATT)". Journal of Pediatric Endocrinology & Metabolism. 23 (10): 999–1009. doi:10.1515/jpem.2010.161. PMID 21158211.
- 1 2 Jameson JL, De Groot LJ (25 February 2015). Edndocrinology: Adult and Pediatric. Elsevier Health Sciences. pp. 2425–2426, 2139. ISBN 978-0-323-32195-2.
- ↑ Elliott S, Latini DM, Walker LM, Wassersug R, Robinson JW (2010). "Androgen deprivation therapy for prostate cancer: recommendations to improve patient and partner quality of life". The Journal of Sexual Medicine. 7 (9): 2996–3010. doi:10.1111/j.1743-6109.2010.01902.x. PMID 20626600.
- ↑ Higano CS (2012). "Sexuality and intimacy after definitive treatment and subsequent androgen deprivation therapy for prostate cancer". Journal of Clinical Oncology. 30 (30): 3720–5. doi:10.1200/JCO.2012.41.8509. PMID 23008326.
- 1 2 3 4 5 van Kemenade JF, Cohen-Kettenis PT, Cohen L, Gooren LJ (1989). "Effects of the pure antiandrogen RU 23.903 (anandron) on sexuality, aggression, and mood in male-to-female transsexuals". Archives of Sexual Behavior. 18 (3): 217–28. doi:10.1007/BF01543196. PMID 2751416.
- 1 2 3 Gooren L, Spinder T, Spijkstra JJ, van Kessel H, Smals A, Rao BR, Hoogslag M (1987). "Sex steroids and pulsatile luteinizing hormone release in men. Studies in estrogen-treated agonadal subjects and eugonadal subjects treated with a novel nonsteroidal antiandrogen". The Journal of Clinical Endocrinology & Metabolism. 64 (4): 763–70. doi:10.1210/jcem-64-4-763. PMID 3102546.
- 1 2 3 de Voogt HJ, Rao BR, Geldof AA, Gooren LJ, Bouman FG (1987). "Androgen action blockade does not result in reduction in size but changes histology of the normal human prostate". Prostate. 11 (4): 305–11. doi:10.1002/pros.2990110403. PMID 2960959.
- ↑ Cohen-Kettenis PT, Gooren LJ (1993). "The Influence of Hormone Treatment on Psychological Functioning of Transsexuals". Journal of Psychology & Human Sexuality. 5 (4): 55–67. doi:10.1300/J056v05n04_04. ISSN 0890-7064.
- ↑ Chang C (22 March 2005). Prostate Cancer: Basic Mechanisms and Therapeutic Approaches. World Scientific. pp. 10–11. ISBN 978-981-4481-61-8. Archived from the original on 8 September 2017.
- ↑ DeVita VT, Lawrence TS, Rosenberg SA (18 March 2016). Prostate and Other Genitourinary Cancers: Cancer: Principles & Practice of Oncology. Wolters Kluwer Health. pp. 1006–. ISBN 978-1-4963-5421-1.
- ↑ Bennett CL, Raisch DW, Sartor O (October 2002). "Pneumonitis associated with nonsteroidal antiandrogens: presumptive evidence of a class effect". Annals of Internal Medicine. 137 (7): 625. doi:10.7326/0003-4819-137-7-200210010-00029. PMID 12353966.
An estimated 0.77% of the 6,480 nilutamide-treated patients, 0.04% of the 41,700 flutamide-treated patients, and 0.01% of the 86,800 bicalutamide-treated patients developed pneumonitis during the study period.
- ↑ Ettner R, Monstrey S, Coleman E (27 May 2016). Principles of Transgender Medicine and Surgery. Routledge. pp. 169–. ISBN 978-1-317-51460-2.
In Europe, the most widely used drug [in the treatment of male-to-female patients] is cyproterone acetate [...]
- ↑ Erickson-Schroth L (12 May 2014). Trans Bodies, Trans Selves: A Resource for the Transgender Community. Oxford University Press. pp. 258–. ISBN 978-0-19-932536-8. Archived from the original on 24 June 2016.
Cyproterone [acetate] is widely used outside the United States as the primary testosterone blocker in transgender women. Cyproterone, however, is not authorized for sale in the United States for any condition and has been associated with liver problems.
- ↑ Azziz R (8 November 2007). Androgen Excess Disorders in Women. Springer Science & Business Media. pp. 382–. ISBN 978-1-59745-179-6.
Cyproterone acetate is an effective treatment for hirsutism and acne [in women] and is widely used throughout the world for this indication.
- ↑ F. William Danby (27 January 2015). Acne: Causes and Practical Management. John Wiley & Sons. pp. 142–. ISBN 978-1-118-23277-4.
- ↑ Loren S Schechter (22 September 2016). Surgical Management of the Transgender Patient. Elsevier Health Sciences. pp. 26–. ISBN 978-0-323-48408-4.
- ↑ H.J.T. Coelingh Benni; H.M. Vemer (15 December 1990). Chronic Hyperandrogenic Anovulation. CRC Press. pp. 152–. ISBN 978-1-85070-322-8.
CPA is not available in the United States. Thus, spironolactone is currently used. [...] In our opinion, [spironolactone] has a weak antiandrogenic activity.
- ↑ Tommaso Falcone; William Hurd (26 April 2007). Clinical Reproductive Medicine and Surgery E-Book. Elsevier Health Sciences. pp. 285–. ISBN 978-0-323-07659-3.
[Flutamide] is also more effective than spironolactone in treating hirsutism, with reduction in hirsutism scores to almost normal after 6 months of therapy with flutamide versus only a 30% reduction in women treated with spironolactone.151
- ↑ Rowland DL, Incrocci L (2 May 2008). Handbook of Sexual and Gender Identity Disorders. John Wiley & Sons. pp. 444–. ISBN 978-0-470-25721-0.
Spironolactone is the most commonly prescribed antiandrogen [for feminizing hormone therapy] in the United States.
- ↑ Thole Z, Manso G, Salgueiro E, Revuelta P, Hidalgo A (2004). "Hepatotoxicity induced by antiandrogens: a review of the literature". Urologia Internationalis. 73 (4): 289–95. doi:10.1159/000081585. PMID 15604569.
- ↑ Kaplowitz N (16 October 2002). Drug-Induced Liver Disease. CRC Press. pp. 618–. ISBN 978-0-203-90912-6.
- ↑ Yun GY, Kim SH, Kim SW, Joo JS, Kim JS, Lee ES, Lee BS, Kang SH, Moon HS, Sung JK, Lee HY, Kim KH (April 2016). "Atypical onset of bicalutamide-induced liver injury". World Journal of Gastroenterology. 22 (15): 4062–5. doi:10.3748/wjg.v22.i15.4062. PMC 4823258. PMID 27099451.
- ↑ Chang S (10 March 2010), Bicalutamide BPCA Drug Use Review in the Pediatric Population (PDF), U.S. Department of Health and Human Service, archived (PDF) from the original on 24 October 2016, retrieved 20 July 2016
- ↑ Emans SJ, Laufer MR (5 January 2012). Emans, Laufer, Goldstein's Pediatric and Adolescent Gynecology. Lippincott Williams & Wilkins. pp. 365–. ISBN 978-1-4511-5406-1. Archived from the original on 16 May 2016.
Therapy with GnRH analogs is expensive and requires intramuscular injections of depot formulations, the insert of a subcutaneous implant yearly, or, much less commonly, daily subcutaneous injections.
- ↑ Hillard PJ (29 March 2013). Practical Pediatric and Adolescent Gynecology. John Wiley & Sons. pp. 182–. ISBN 978-1-118-53857-9.
Treatment is expensive, with costs typicall in the range of $10,000–$15,000 per year.
- ↑ Styne DM (25 April 2016). "Disorders of Puberty". Pediatric Endocrinology: A Clinical Handbook. Springer. pp. 197–. ISBN 978-3-319-18371-8.
Antiandrogens are used [...] in conditions such as premature Leydig cell and germ cell maturation in boys to decrease androgen effects if the source of androgens cannot be removed.
- ↑ Lenz AM, Shulman D, Eugster EA, Rahhal S, Fuqua JS, Pescovitz OH, Lewis KA (September 2010). "Bicalutamide and third-generation aromatase inhibitors in testotoxicosis". Pediatrics. 126 (3): e728–33. doi:10.1542/peds.2010-0596. PMC 4096839. PMID 20713483.
- ↑ Levey HR, Kutlu O, Bivalacqua TJ (2012). "Medical management of ischemic stuttering priapism: a contemporary review of the literature". Asian Journal of Andrology. 14 (1): 156–63. doi:10.1038/aja.2011.114. PMC 3753435. PMID 22057380.
- ↑ Broderick GA, Kadioglu A, Bivalacqua TJ, Ghanem H, Nehra A, Shamloul R (2010). "Priapism: pathogenesis, epidemiology, and management". The Journal of Sexual Medicine. 7 (1 Pt 2): 476–500. doi:10.1111/j.1743-6109.2009.01625.x. PMID 20092449.
- 1 2 3 Chow K, Payne S (2008). "The pharmacological management of intermittent priapismic states". BJU International. 102 (11): 1515–21. doi:10.1111/j.1464-410X.2008.07951.x. PMID 18793304.
- 1 2 3 Dahm P, Rao DS, Donatucci CF (2002). "Antiandrogens in the treatment of priapism". Urology. 59 (1): 138. doi:10.1016/S0090-4295(01)01492-3. PMID 11796309.
- 1 2 3 Yuan J, Desouza R, Westney OL, Wang R (2008). "Insights of priapism mechanism and rationale treatment for recurrent priapism". Asian Journal of Andrology. 10 (1): 88–101. doi:10.1111/j.1745-7262.2008.00314.x. PMID 18087648.
- ↑ Dart RC (2004). Medical Toxicology. Lippincott Williams & Wilkins. pp. 497, 521. ISBN 978-0-7817-2845-4. Archived from the original on 11 May 2016.
- ↑ Skidmore-Roth L (17 April 2013). Mosby's 2014 Nursing Drug Reference – Elsevieron VitalSource. Elsevier Health Sciences. pp. 193–194. ISBN 978-0-323-22267-9.
- ↑ m Sternberg MH, Forget BG, Higgs DR, Weatherall DJ (17 August 2009). Disorders of Hemoglobin: Genetics, Pathophysiology, and Clinical Management. Cambridge University Press. pp. 476–. ISBN 978-1-139-48080-2. Archived from the original on 24 August 2017.
- 1 2 3 4 5 6 Gooren LJ (2011). "Clinical review: Ethical and medical considerations of androgen deprivation treatment of sex offenders". The Journal of Clinical Endocrinology & Metabolism. 96 (12): 3628–37. doi:10.1210/jc.2011-1540. PMID 21956411.
- 1 2 3 Houts FW, Taller I, Tucker DE, Berlin FS (2011). "Androgen deprivation treatment of sexual behavior". Advances in Psychosomatic Medicine. 31: 149–63. doi:10.1159/000330196. PMID 22005210.
- 1 2 3 Giltay EJ, Gooren LJ (2009). "Potential side effects of androgen deprivation treatment in sex offenders". The Journal of the American Academy of Psychiatry and the Law. 37 (1): 53–8. PMID 19297634.
- ↑ Khan O, Mashru A (2016). "The efficacy, safety and ethics of the use of testosterone-suppressing agents in the management of sex offending". Current Opinion in Endocrinology, Diabetes and Obesity. 23 (3): 271–8. doi:10.1097/MED.0000000000000257. PMID 27032060.
- ↑ Dangerous Sex Offenders: A Task Force Report of the American Psychiatric Association. American Psychiatric Pub. 1999. pp. 111–. ISBN 978-0-89042-280-9.
- ↑ Wadhwa VK, Weston R, Parr NJ (2011). "Bicalutamide monotherapy preserves bone mineral density, muscle strength and has significant health-related quality of life benefits for osteoporotic men with prostate cancer". BJU International. 107 (12): 1923–9. doi:10.1111/j.1464-410X.2010.09726.x. PMID 20950306.
- ↑ Rousseau L, Couture M, Dupont A, Labrie F, Couture N (1990). "Effect of combined androgen blockade with an LHRH agonist and flutamide in one severe case of male exhibitionism". The Canadian Journal of Psychiatry. 35 (4): 338–41. doi:10.1177/070674379003500412. PMID 2189544.