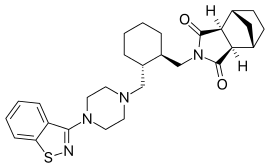
Lurasidone
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Latuda |
| Synonyms | SM-13,496 |
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a611016 |
| License data |
|
| Pregnancy category | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 9–19% (oral)[1] |
| Protein binding | ~99%[2] |
| Metabolism | Liver (CYP3A4-mediated)[1] |
| Elimination half-life | 18–40 hours[1][2] |
| Excretion |
Faecal (67–80%), renal (9–19%)[1][2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard |
100.225.187 |
| Chemical and physical data | |
| Formula | C28H36N4O2S |
| Molar mass | 492.68 g/mol |
| 3D model (JSmol) | |
| Specific rotation | [α]20D −59° |
| Melting point | 176 to 178 °C (349 to 352 °F) |
| Solubility in water | 45 mg/mL (20 °C) |
| |
| |
Lurasidone (/ljʊəˈræsɪdoʊn/; trade name Latuda) is an atypical antipsychotic developed by Dainippon Sumitomo Pharma[3] and marketed by Sunovion in the U.S. It has been an FDA approved treatment for schizophrenia since 2010 and for treating depressive episodes in adults with bipolar I disorder since 2013. It can be used alone or in combination with mood stabilizers such as lithium or valproate (e.g., Depakote).
Medical uses
Lurasidone is approved by the US Food and Drug Administration (FDA) for the treatment of schizophrenia since 2010[4] and depressive episodes associated with bipolar I disorder since 2013.[5] It received regulatory approval in the United Kingdom in September 2014. In October 2014, NHS Scotland advised use of lurasidone for schizophrenic adults who have not seen improvements with previous antipsychotics due to problems that arise from weight gain or changes in metabolic pathways when taking other medications.[6] It received approval by the European Medicines Agency on 24 January 2014. It was launched in Canada for the treatment of schizophrenia in September 2012, Health Canada giving their Summary Basis of Decision (SBD) as favourable on 15 October 2012.[7] European Commission has granted a marketing authorization for once-daily oral lurasidone for the treatment of schizophrenia in adults.[8] It is approved for use in the EU.[9]
In a 2013 study in a comparison of 15 antipsychotic drugs in effectiveness in treating schizophrenic symptoms, lurasidone demonstrated mild effectiveness. As effective as iloperidone, and 13 to 15% less effective than ziprasidone, chlorpromazine, and asenapine.[10]
In July 2013 lurasidone received approval for bipolar I depression.[5] Few available atypical antipsychotics are known to possess antidepressant efficacy in bipolar disorder (with the notable exceptions being quetiapine,[11][12][13][14] olanzapine[15][16][17] and possibly asenapine[18]) as a monotherapy, even though the majority of atypical antipsychotics are known to possess significant antimanic activity,[19] which is yet to be clearly demonstrated for lurasidone.
Lurasidone is not approved by the Food and Drug Administration (FDA) for the treatment of behavior disorders in older adults with dementia.[20]
Contraindications
Lurasidone is contraindicated in individuals who are taking strong inhibitors of the liver enzyme CYP3A4 (ketoconazole, clarithromycin, ritonavir, levodropropizine, etc.) or inducers (carbamazepine, St. John's wort, phenytoin, rifampicin etc.).[21] The use of lurasidone in pregnant women has not been studied and is not recommended; in animal studies, no risks have been found.[22] Excretion in breast milk is also unknown; lurasidone is not recommended for breastfeeding women.[23] In the United States it is not indicated for use in children.[24]
Side effects
Side effects are generally similar to other antipsychotics. The drug has a relatively well-tolerated side effect profile, with low propensity for QTc interval changes, weight gain and lipid-related adverse effects.[25] In a 2013 meta-analysis of the efficacy and tolerability of 15 antipsychotic drugs it was found to produce the second least (after haloperidol) weight gain, the least QT interval prolongation, the fourth most extrapyramidal side effects (after haloperidol, zotepine and chlorpromazine) and the sixth least sedation (after paliperidone, sertindole, amisulpride, iloperidone and aripiprazole).[10]
As with other atypical neuroleptics, lurasidone should be used with caution in the elderly because it puts them at an increased risk for a stroke or transient ischemic attack;[26][27] however, these risks are not likely to be greater than those associated with antipsychotics of other classes.[28] Similarly, lurasidone should not be used to treat dementia-related psychosis, as evidence has shown increased mortality with antipsychotic use.[29]
Weight gain is reported in up to 15 and 16 percent of users.[30][31]
Interactions
Blood plasma concentrations may be increased when combined with CYP3A4 inhibitors, possibly leading to more side effects. This has been clinically verified for ketoconazole, which increases lurasidone exposure by a factor of 9, and is also expected for other 3A4 inhibitors such as grapefruit juice. Co-administration of CYP3A4 inducers like rifampicin or St. John's wort can reduce plasma levels of lurasidone and its active metabolite, and consequently decrease the effects of the drug. For rifamipcin, the reduction was sixfold in a study.[2]
Pharmacology
Pharmacodynamics
| Site | Ki (nM) | Action | Species | Ref |
|---|---|---|---|---|
| 5-HT1A | 6.75 | Partial agonist | Rat | [33] |
| 5-HT2A | 2.03 | Antagonist | Rat | [33] |
| 5-HT2B | ND | ND | ND | ND |
| 5-HT2C | 415 | ND | Pig | [33] |
| 5-HT3 | >1,000 | ND | ND | [33] |
| 5-HT4 | >1,000 | ND | ND | [33] |
| 5-HT7 | 0.495 | Antagonist | Human | [33] |
| D1 | 262 | ND | ND | [33] |
| D2 | 1.68 | Antagonist | Rat | [33] |
| D3 | 15.7 | Antagonist | ND | ND |
| D4.4 | 30 | ND | ND | ND |
| D5 | ND | ND | ND | ND |
| α1 | 47.9 | ND | Rat | [33] |
| α2A | 40.7 | ND | Human | [33] |
| α2B | ND | ND | ND | ND |
| α2C | 10.8 | Antagonist | Human | [33] |
| β1 | >1,000 | ND | ND | [33] |
| β2 | >1,000 | ND | ND | [33] |
| H1 | >1,000 | ND | Guinea pig | [33] |
| M1 | >1,000 | ND | Human | [33] |
| SERT | >1,000 | ND | ND | [33] |
| NET | ND | ND | ND | ND |
| DAT | >1,000 | ND | ND | [33] |
| Values are Ki (nM). The smaller the value, the more strongly the drug binds to the site. | ||||
Lurasidone acts as an antagonist of the dopamine D2 and D3 receptors, the serotonin 5-HT2A and 5-HT7 receptors, and the α2C-adrenergic receptor, and as a partial agonist of the serotonin 5-HT1A receptor.[34][33] It has only low and likely clinically unimportant affinity for the serotonin 5-HT2C receptor, which may underlie its low propensity for appetite stimulation and weight gain.[33][35][36] The drug also has negligible affinity for the histamine H1 receptor and the muscarinic acetylcholine receptors, and hence has no antihistamine or anticholinergic effects.[34][37]
Pharmacokinetics
Lurasidone is taken by mouth and has an estimated absorption rate of 9 to 19%.[1] Studies have shown that when lurasidone is taken with food, absorption increases about twofold. Peak blood plasma concentrations are reached after one to three hours. About 99% of the circulating substance are bound to plasma proteins.[2]
Lurasidone is mainly metabolized in the liver via the enzyme CYP3A4, but has negligible affinity to other cytochrome P450 enzymes. It is transported by P-glycoprotein and ABCG2 and also inhibits these carrier proteins in vitro. It also inhibits the solute carrier protein SLC22A1, but no other relevant transporters.[2][26]
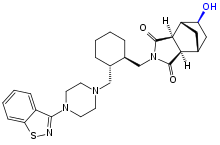
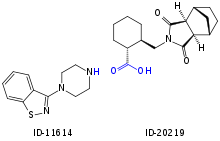
Main metabolism pathways are oxidative N-dealkylation between the piperazine and cyclohexane rings, hydroxylation of the norbornane ring, and S-oxidation.[2][40]:59 Other pathways are hydroxylation of the cyclohexane ring and reductive cleavage of the isothiazole ring followed by S-methylation.[39] The two relevant active metabolites are the norbornane hydroxylation products called ID-14283 and ID-14326, the former reaching pharmacologically relevant blood plasma concentrations. The two major inactive metabolites are the N-dealkylation products (the carboxylic acid ID-20219 and the piperazine ID-11614[39]), and a norbornane hydroxylated derivative of ID-20219 (ID-20220). Of lurasidone and its metabolites circulating in the blood, the native drug makes up 11%, the main active metabolite 4%, and the inactive carboxylic acids 24% and 11%, respectively.[1][2] Several dozen metabolites have been identified altogether.[40]:59–61
Biological half-life is given as 18 hours or 20 to 40 hours in different sources. 80% or 67% of a radiolabelled dose was recovered from the faeces, and 9% or 19% from the urine.[1][2]
History
Lurasidone was synthesized in 2003.[41]
Lurasidone is a structural analogue of Ziprasidone (Zeldox). Lurasidone shows a very close pharmacological profile and has been synthesized similarly to Ziprasidone.[42]
Lurasidone is chemically similar to Perospirone (also a chemical analogue of Zeldox), as well as Risperidone, Paliperidone and Iloperidone.[43]
The FDA approved the drug in 2010.[40]
Society and culture
Trade names
In India, this drug is available under the brand names of Atlura, Lurace, Lurafic, Luramax, Lurasid, Lurastar, Latuda, Lurata[44] and additionally as Alsiva, Emsidon, Lurakem, Luratrend, Tablura, and Unison.[45]
Research
Lurasidone may be useful for treating the cognitive and memory deficits seen in schizophrenia. In animal studies, it reversed dizocilpine-induced learning and memory impairment and was found to be superior in doing this to all of the other antipsychotics examined, including risperidone, olanzapine, quetiapine, clozapine, aripiprazole, and haloperidol.[34][46] Lurasidone has activity at several serotonin receptors that are involved in learning and memory, and unlike most other antipsychotics, lacks any anticholinergic effects (which are known to impair cognitive processes and memory).[34] These properties may underlie its improved effectiveness in treating these symptoms relative to older agents.[34]
See also
References
- 1 2 3 4 5 6 7 "Product information Latuda (lurasidone hydrochloride)" (PDF). TGA eBusiness Services. Therapeutic Goods Administration. 16 April 2014. Retrieved 1 May 2014.
- 1 2 3 4 5 6 7 8 9 "Latuda: EPAR – Product Information" (PDF). European Medicines Agency. 14 April 2016.
- ↑ Meyer, Jonathan M; Loebel, Antony D; Schweizer, Edward (2009). "Lurasidone: A new drug in development for schizophrenia". Expert Opinion on Investigational Drugs. 18 (11): 1715–26. doi:10.1517/13543780903286388. PMID 19780705.
- ↑ "FDA approves Latuda to treat schizophrenia in adults" (Press release). USFDA. 2010-10-28. Retrieved October 29, 2010.
- 1 2 Lowes R. Lurasidone Approved for Bipolar Depression [Internet]. Medscape. 2013 [cited 2013 Oct 2]. Available from: http://www.medscape.com/viewarticle/807204
- ↑ "Lurasidone, 18.5mg, 37mg, 74mg film-coated tablets (Latuda) SMC No. (994/14)" (PDF). Scottish Medicines Consortium. 2014.
- ↑ "Summary Basis of Decision (SBD) for Latuda". Health Canada. 2012.
- ↑ "European Marketing Authorization for Latuda". takeda.com. Retrieved 25 November 2015.
- ↑ "European Medicines Agency - Find medicine - Latuda". europa.eu. Retrieved 25 November 2015.
- 1 2 Leucht, Stefan; Cipriani, Andrea; Spineli, Loukia; Mavridis, Dimitris; Örey, Deniz; Richter, Franziska; Samara, Myrto; Barbui, Corrado; Engel, Rolf R; Geddes, John R; Kissling, Werner; Stapf, Marko Paul; Lässig, Bettina; Salanti, Georgia; Davis, John M (2013). "Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis". The Lancet. 382 (9896): 951–62. doi:10.1016/S0140-6736(13)60733-3. PMID 23810019.
- ↑ Young, Allan H.; McElroy, Susan L.; Bauer, Michael; Philips, Nabil; Chang, William; Olausson, Bengt; Paulsson, Björn; Brecher, Martin; EMBOLDEN I (Trial 001) Investigators (2010). "A Double-Blind, Placebo-Controlled Study of Quetiapine and Lithium Monotherapy in Adults in the Acute Phase of Bipolar Depression (EMBOLDEN I)". The Journal of Clinical Psychiatry. 71 (2): 150–62. doi:10.4088/JCP.08m04995gre. PMID 20122369.
- ↑ Suppes, Trisha; Datto, Catherine; Minkwitz, Margaret; Nordenhem, Arvid; Walker, Chris; Darko, Denis (2010). "Effectiveness of the extended release formulation of quetiapine as monotherapy for the treatment of acute bipolar depression". Journal of Affective Disorders. 121 (1–2): 106–15. doi:10.1016/j.jad.2009.10.007. PMID 19903574.
- ↑ "Corrigendum". Bipolar Disorders. 10 (3): 451. 2008. doi:10.1111/j.1399-5618.2008.00585.x.
- ↑ Thase, ME (2008). "Quetiapine monotherapy for bipolar depression". Neuropsychiatric Disease and Treatment. 4 (1): 11–21. doi:10.2147/ndt.s1162. PMC 2515925. PMID 18728771.
- ↑ Tohen, Mauricio; Vieta, E; Calabrese, J; Ketter, TA; Sachs, G; Bowden, C; Mitchell, PB; Centorrino, F; Risser, R; Baker, RW; Evans, AR; Beymer, K; Dube, S; Tollefson, GD; Breier, A (2003). "Efficacy of Olanzapine and Olanzapine-Fluoxetine Combination in the Treatment of Bipolar I Depression". Archives of General Psychiatry. 60 (11): 1079–88. doi:10.1001/archpsyc.60.11.1079. PMID 14609883.
- ↑ Tohen, M.; Katagiri, H.; Fujikoshi, S.; Kanba, S. (2013). "Efficacy of olanzapine monotherapy in acute bipolar depression: A pooled analysis of controlled studies". Journal of Affective Disorders. 149 (1–3): 196–201. doi:10.1016/j.jad.2013.01.022. PMID 23485111.
- ↑ Corya, Sara A.; Perlis, Roy H.; Keck Jr, Paul E.; Lin, Daniel Y.; Case, Michael G.; Williamson, Doug J.; Tohen, Mauricio F. (2006). "A 24-Week Open-Label Extension Study of Olanzapine-Fluoxetine Combination and Olanzapine Monotherapy in the Treatment of Bipolar Depression". The Journal of Clinical Psychiatry. 67 (5): 798–806. doi:10.4088/JCP.v67n0514. PMID 16841630.
- ↑ Azorin, J.M.; Sapin, C.; Weiller, E. (2013). "Effect of asenapine on manic and depressive symptoms in bipolar I patients with mixed episodes: Results from post hoc analyses". Journal of Affective Disorders. 145 (1): 62–9. doi:10.1016/j.jad.2012.07.013. PMID 22868059.
- ↑ Cipriani, Andrea; Barbui, Corrado; Salanti, Georgia; Rendell, Jennifer; Brown, Rachel; Stockton, Sarah; Purgato, Marianna; Spineli, Loukia M; Goodwin, Guy M; Geddes, John R (2011). "Comparative efficacy and acceptability of antimanic drugs in acute mania: A multiple-treatments meta-analysis". The Lancet. 378 (9799): 1306–15. doi:10.1016/S0140-6736(11)60873-8. PMID 21851976.
- ↑ "Lurasidone: MedlinePlus Drug Information". medlineplus.gov. Retrieved 2018-09-11.
- ↑ Chiu, Yu-Yuan; Ereshefsky, Larry; Preskorn, Sheldon H.; Poola, Nagaraju; Loebel, Antony (2014). "Lurasidone drug-drug interaction studies: a comprehensive review". Drug Metabolism and Drug Interactions. 29 (3): 191–202. doi:10.1515/dmdi-2014-0005. PMID 24825095.
- ↑ Pregnancy category
- ↑ ACOG Committee on Practice Bulletins--Obstetrics. (2008). "ACOG Practice Bulletin No. 92: Use of Psychiatric Medications During Pregnancy and Lactation". Obstetrics & Gynecology. 111 (4): 1001–20. doi:10.1097/AOG.0b013e31816fd910. PMID 18378767.
- ↑ Moore, Troy (2011). "Schizophrenia Treatment Guidelinesin the United States". Clinical Schizophrenia & Related Psychoses. 5 (1): 40–9. doi:10.3371/CSRP.5.1.6. PMID 21459738.
- ↑ "Lurasidone Demonstrated Efficacy in Treating Patients With Schizophrenia in Pivotal Phase 3 Study" (Press release). Dainippon Sumitomo Pharma. Aug 26, 2009. Retrieved October 3, 2016.
- 1 2 "Latuda: Prescribing Information". Psychotherapeutic Drugs. Retrieved 2010-12-17.
- ↑ "Latuda". Drugs.com. Retrieved 2010-12-17.
- ↑ Herrmann, Nathan; Mamdani, Muhammad; Lanctôt, Krista L. (2004). "Atypical Antipsychotics and Risk of Cerebrovascular Accidents". American Journal of Psychiatry. 161 (6): 1113–5. doi:10.1176/appi.ajp.161.6.1113. PMID 15169702.
- ↑ Sunovion Pharmaceuticals. "Latuda Prescribing Information" (PDF). Latuda.com. Retrieved 25 March 2014.
- ↑ Meyer, Jonathan M.; Mao, Yongcai; Pikalov, Andrei; Cucchiaro, Josephine; Loebel, Antony (2015). "Weight change during long-term treatment with lurasidone". International Clinical Psychopharmacology. 30 (6): 342–50. doi:10.1097/YIC.0000000000000091. PMC 4593468. PMID 26196189.
- ↑ Ketter, Terence A.; Sarma, Kaushik; Silva, Robert; Kroger, Hans; Cucchiaro, Josephine; Loebel, Antony (2016). "Lurasidone in the long-term treatment of patients with bipolar disorder: A 24-week open-label extension study". Depression and Anxiety. 33 (5): 424–34. doi:10.1002/da.22479. PMC 5069590. PMID 26918425. Lay summary – NEJM Journal Watch (March 14, 2016).
- ↑ Roth, BL; Driscol, J. "PDSP Ki Database" (HTML). Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 14 August 2017.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 Ishibashi T, Horisawa T, Tokuda K, Ishiyama T, Ogasa M, Tagashira R, Matsumoto K, Nishikawa H, Ueda Y, Toma S, Oki H, Tanno N, Saji I, Ito A, Ohno Y, Nakamura M (2010). "Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity". J. Pharmacol. Exp. Ther. 334 (1): 171–81. doi:10.1124/jpet.110.167346. PMID 20404009.
- 1 2 3 4 5 Ishiyama, Takeo; Tokuda, Kumiko; Ishibashi, Tadashi; Ito, Akira; Toma, Satoko; Ohno, Yukihiro (2007). "Lurasidone (SM-13496), a novel atypical antipsychotic drug, reverses MK-801-induced impairment of learning and memory in the rat passive-avoidance test". European Journal of Pharmacology. 572 (2–3): 160–70. doi:10.1016/j.ejphar.2007.06.058. PMID 17662268.
- ↑ Samalin, L.; Ben Gharbia, M.; Garnier, M.; Llorca, P.-M. (2014). "Efficacité et tolérance à court terme de la lurasidone dans la prise en charge de la schizophrénie" [Short-term efficacy and safety of lurasidone in the treatment of schizophrenia]. L'Encéphale (in French). 40 (6): 507–17. doi:10.1016/j.encep.2014.10.009. PMID 25453735.
- ↑ Lincoln, Jana; Tripathi, Aveekshit (2011). "Lurasidone for Schizophrenia". Current Psychiatry. 10 (1): 67–70.
- ↑ Greenberg, W. M.; Citrome, L (2016). "Pharmacokinetics and Pharmacodynamics of Lurasidone Hydrochloride, a Second-Generation Antipsychotic: A Systematic Review of the Published Literature". Clinical Pharmacokinetics. 56 (5): 493–503. doi:10.1007/s40262-016-0465-5. PMID 27722855.
- ↑ Katteboina, Mahitej Yadav; Pilli, Nageswara Rao; Mullangi, Ramesh; Seelam, Raghunadha Reddy; Satla, Shobha Rani (2016). "LC-MS/MS assay for the determination of lurasidone and its active metabolite, ID-14283 in human plasma and its application to a clinical pharmacokinetic study". Biomedical Chromatography. 30 (7): 1065–74. doi:10.1002/bmc.3651. PMID 26577488.
- 1 2 3 4 Caccia, Silvio; Pasina; Nobili, Alessandro (2012). "Critical appraisal of lurasidone in the management of schizophrenia". Neuropsychiatric Disease and Treatment. 8: 155–68. doi:10.2147/NDT.S18059. PMC 3346058. PMID 22570547.
- 1 2 3 4 "Lurasidone pharmacology review" (PDF). Center for Drug Evaluation and Research. 30 December 2009.
- ↑ "Leading Latuda through the crowd". 1 October 2011.
- ↑ Vardanyan, Ruben; Hruby, Victor (7 January 2016). "Synthesis of Best-Seller Drugs". Academic Press – via Google Books.
- ↑ "Wayback Machine" (PDF). 17 November 2016.
- ↑ "'Lurasidone' drug search". CIMS India. Retrieved 2018-04-21.
- ↑ "Generic Drugs (ndrugs.com)". Retrieved 2018-04-30.
- ↑ Enomoto, T; Ishibashi, T; Tokuda, K; Ishiyama, T; Toma, S; Ito, A (2008). "Lurasidone reverses MK-801-induced impairment of learning and memory in the Morris water maze and radial-arm maze tests in rats". Behavioural Brain Research. 186 (2): 197–207. doi:10.1016/j.bbr.2007.08.012. PMID 17881065.