Methylation
In the chemical sciences, methylation denotes the addition of a methyl group on a substrate, or the substitution of an atom (or group) by a methyl group. Methylation is a form of alkylation, with a methyl group, rather than a larger carbon chain, replacing a hydrogen atom. These terms are commonly used in chemistry, biochemistry, soil science, and the biological sciences.
In biological systems, methylation is catalyzed by enzymes; such methylation can be involved in modification of heavy metals, regulation of gene expression, regulation of protein function, and RNA processing. In vitro methylation of tissue samples is also one method for reducing certain histological staining artifacts. The counterpart of methylation is called demethylation.
In biology
In biological systems, methylation is accomplished by enzymes; methylation can modify heavy metals, regulate gene expression, RNA processing and protein function. It has been recognized as a key process underlying epigenetics.
Methanogenesis
Methanogenesis, the process that generates methane, is the result of a series of methylation reactions. These reactions are affected by a set of enzymes harbored by a family of anaerobic microbes.[1]
In reverse methanogenesis, methane serves as the methylating agent.
O-Methyltransferases
A wide variety of phenols undergo O-methylation to give anisole derivatives. This process, catalyzed by enzymes such as caffeoyl-CoA O-methyltransferase, is a key reaction in the biosynthesis of lignols, percursors to lignin, a major structural component of plants.
5-O-methylations
Plants produce flavonoids and isoflavones with methylations on hydroxyl groups, i.e. methoxy bonds. This 5-O-methylation affects the flavonoid´s water solubility. Examples are 5-O-methylgenistein, 5-O-methylmyricetin or 5-O-methylquercetin, also known as azaleatin.
Methionine synthase
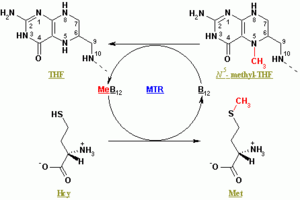
Methionine synthase regenerates methionine (Met) from homocysteine (Hcy). The overall reaction transforms 5-methyltetrahydrofolate (N5-MeTHF) into tetrahydrofolate (THF) while transferring a methyl group to Hcy to form Met. Methionine Synthases can be cobalamin-dependent and cobalamin-independent: Plants have both, animals depend on the methylcobalamin-dependent form.
In methylcobalamin-dependent forms of the enzyme, the reaction proceeds by two steps in a ping-pong reaction. The enzyme is initially primed into a reactive state by the transfer of a methyl group from N5-MeTHF to Co(I) in enzyme-bound cobalamin (Cob), forming methyl-cobalamin(Me-Cob) that now contains Me-Co(III) and activating the enzyme. Then, a Hcy that has coordinated to an enzyme-bound zinc to form a reactive thiolate reacts with the Me-Cob. The activated methyl group is transferred from Me-Cob to the Hcy thiolate, which regenerates Co(I) in Cob, and Met is released from the enzyme.[2]
Heavy metals: arsenic, mercury, cadmium
Biomethylation is the pathway for converting some heavy elements into more mobile or more lethal derivatives that can enter the food chain. The biomethylation of arsenic compounds starts with the formation of methanearsonates. Thus, trivalent inorganic arsenic compounds are methylated to give methanearsonate. S-adenosylmethionine is the methyl donor. The methanearsonates are the precursors to dimethylarsonates, again by the cycle of reduction (to methylarsonous acid) followed by a second methylation.[3] Related pathways apply to the biosynthesis of methylmercury.
Epigenetic methylation
DNA/RNA methylation
DNA methylation in vertebrates typically occurs at CpG sites (cytosine-phosphate-guanine sites–that is, where a cytosine is directly followed by a guanine in the DNA sequence). This methylation results in the conversion of the cytosine to 5-methylcytosine. The formation of Me-CpG is catalyzed by the enzyme DNA methyltransferase. Human DNA has about 80–90% of CpG sites methylated, but there are certain areas, known as CpG islands, that are GC-rich (high guanine and cytosine content, made up of about 65% CG residues), wherein none are methylated. These are associated with the promoters of 56% of mammalian genes, including all ubiquitously expressed genes. One to two percent of the human genome are CpG clusters, and there is an inverse relationship between CpG methylation and transcriptional activity. Methylation contributing to epigenetic inheritance can occur through either DNA methylation or protein methylation. Similarly, RNA methylation occurs in different RNA species viz. tRNA, rRNA, mRNA, tmRNA, snRNA, snoRNA, miRNA, and viral RNA. Different catalytic strategies are employed for RNA methylation by a variety of RNA-methyltransferases. RNA methylation is thought to have existed before DNA methylation in the early forms of life evolving on earth.[4]
N6-methyladenosine (m6A) is the most common and abundant methylation modification in RNA molecules (mRNA) present in eukaryotes. 5-methylcytosine (5-mC) also commonly occurs in various RNA molecules. Recent data strongly suggest that m6A and 5-mC RNA methylation affects the regulation of various biological processes such as RNA stability and mRNA translation,[5] and that abnormal RNA methylation contributes to etiology of human diseases.[6]
Protein methylation
Protein methylation typically takes place on arginine or lysine amino acid residues in the protein sequence.[7] Arginine can be methylated once (monomethylated arginine) or twice, with either both methyl groups on one terminal nitrogen (asymmetric dimethylarginine) or one on both nitrogens (symmetric dimethylarginine), by protein arginine methyltransferases (PRMTs). Lysine can be methylated once, twice, or three times by lysine methyltransferases. Protein methylation has been most studied in the histones. The transfer of methyl groups from S-adenosyl methionine to histones is catalyzed by enzymes known as histone methyltransferases. Histones that are methylated on certain residues can act epigenetically to repress or activate gene expression.[8][9] Protein methylation is one type of post-translational modification.
In chemistry
The term methylation in organic chemistry refers to the alkylation process used to describe the delivery of a CH3 group.[10]
Electrophilic methylation
Methylations are commonly performed using electrophilic methyl sources such as iodomethane,[11] dimethyl sulfate,[12][13] dimethyl carbonate,[14] or tetramethylammonium chloride.[15] Less common but more powerful (and more dangerous) methylating reagents include methyl triflate,[16] diazomethane,[17] and methyl fluorosulfonate (magic methyl). These reagents all react via SN2 nucleophilic substitutions. For example, a carboxylate may be methylated on oxygen to give a methyl ester; an alkoxide salt RO− may be likewise methylated to give an ether, ROCH3; or a ketone enolate may be methylated on carbon to produce a new ketone.
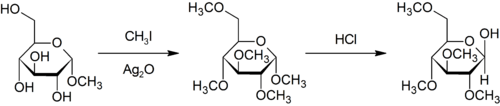
The Purdie methylation is a specific for the methylation at oxygen of carbohydrates using iodomethane and silver oxide.[18]
Eschweiler-Clarke methylation
The Eschweiler-Clarke reaction is a method for methylation of amines.[19] This method avoids the risk of quaternization, which occurs when amines are methylated with methyl halides.
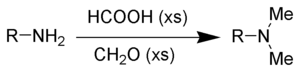
Diazomethane and trimethylsilyldiazomethane
Diazomethane and the safer analogue trimethylsilyldiazomethane methylate carboxylic acids, phenols, and even alcohols:
- RCO2H + tmsCHN2 + CH3OH → RCO2CH3 + CH3Otms + N2
The method offers the advantage that the side products are easily removed from the product mixture.[20]
Nucleophilic methylation
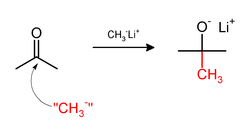
Methylation sometimes involve use of nucleophilic methyl reagents. Strongly nucleophilic methylating agents include methyllithium (CH3Li)[21] or Grignard reagents such as methylmagnesium bromide (CH3MgX).[22] For example, CH3Li will add methyl groups to the carbonyl (C=O) of ketones and aldehyde.:
Milder methylating agents include tetramethyltin, dimethylzinc, and trimethylaluminium.[23]
See also
Biology topics
- Bisulfite sequencing – the biochemical method used to determine the presence or absence of methyl groups on a DNA sequence
- MethDB DNA Methylation Database
- Microscale thermophoresis – a biophysical method to determine the methylisation state of DNA[24]
Organic chemistry topics
References
- ↑ Thauer, R. K., "Biochemistry of Methanogenesis: a Tribute to Marjory Stephenson", Microbiology, 1998, volume 144, pages 2377-2406.
- ↑ Matthews, R. G.; Smith, A. E.; Zhou, Z. S.; Taurog, R. E.; Bandarian, V.; Evans, J. C.; Ludwig, M. (2003). "Cobalamin-Dependent and Cobalamin-Independent Methionine Synthases: Are There Two Solutions to the Same Chemical Problem?". Helvetica Chimica Acta. 86 (12): 3939–3954. doi:10.1002/hlca.200390329.
- ↑ Styblo, M.; Del Razo, L. M.; Vega, L.; Germolec, D. R.; LeCluyse, E. L.; Hamilton, G. A.; Reed, W.; Wang, C.; Cullen, W. R.; Thomas, D. J. (2000). "Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells". Archives of Toxicology. 74 (6): 289–299. doi:10.1007/s002040000134.
- ↑ Rana, Ajay K.; Ankri, Serge (2016-01-01). "Reviving the RNA World: An Insight into the Appearance of RNA Methyltransferases". Front Genet. 7: 99. doi:10.3389/fgene.2016.00099. PMC 4893491. PMID 27375676.
- ↑ Choi, Junhong; Ieong, Ka-Weng; Demirci, Hasan; Chen, Jin; Petrov, Alexey; Prabhakar, Arjun; O'Leary, Seán E.; Dominissini, Dan; Rechavi, Gideon (February 2016). "N6-methyladenosine in mRNA disrupts tRNA selection and translation-elongation dynamics". Nature Structural & Molecular Biology. 23 (2): 110–115. doi:10.1038/nsmb.3148. ISSN 1545-9993. PMC 4826618. PMID 26751643.
- ↑ Stewart, Kendal (15 September 2017). "Methylation (MTHFR) Testing & Folate Deficiency". Retrieved 11 October 2017.
- ↑ Walsh, Christopher (2006). "Chapter 5 – Protein Methylation" (PDF). Posttranslational modification of proteins: expanding nature's inventory. Roberts and Co. Publishers. ISBN 0-9747077-3-2.
- ↑ Grewal, S. I.; Rice, J. C. (2004). "Regulation of heterochromatin by histone methylation and small RNAs". Current Opinion in Cell Biology. 16 (3): 230–238. doi:10.1016/j.ceb.2004.04.002. PMID 15145346.
- ↑ Nakayama, J. -I.; Rice, J. C.; Strahl, B. D.; Allis, C. D.; Grewal, S. I. (2001). "Role of Histone H3 Lysine 9 Methylation in Epigenetic Control of Heterochromatin Assembly". Science. 292 (5514): 110–113. Bibcode:2001Sci...292..110N. doi:10.1126/science.1060118. PMID 11283354.
- ↑ March, Jerry; Smith, Michael W (2001). March's advanced organic chemistry: reactions, mechanisms, and structure. New York: Wiley. ISBN 0-471-58589-0.
- ↑ Vyas, G. N.; Shah, N. M. (1951). "Quninacetophenone monomethyl ether". Organic Syntheses. 31: 90. doi:10.15227/orgsyn.031.0090.
- ↑ Hiers, G. S. (1929). "Anisole". Organic Syntheses. 9: 12. doi:10.15227/orgsyn.009.0012.
- ↑ Icke, Roland N.; Redemann, Ernst; Wisegarver, Burnett B.; Alles, Gordon A. (1949). "m-Methoxybenzaldehyde". Organic Syntheses. 29: 63. doi:10.15227/orgsyn.029.0063.
- ↑ Tundo, Pietro; Selva, Maurizio; Bomben, Andrea (1999). "Mono-C-methylathion of arylacetonitriles and methyl arylacetates by dimethyl carbonate: a general method for the synthesis of pure 2-arylpropionic acids. 2-Phenylpropionic acid". Organic Syntheses. 76: 169. doi:10.15227/orgsyn.076.0169.
- ↑ Nenad, Maraš; Polanc, Slovenko; Kočevar, Marijan (2008). "Microwave-assisted methylation of phenols with tetramethylammonium chloride in the presence of K2CO3 or Cs2CO3". Tetrahedron. 64 (51): 11618–11624. doi:10.1016/j.tet.2008.10.024.
- ↑ Poon, Kevin W. C.; Albiniak, Philip A.; Dudley, Gregory B. (2007). "Protection of alcohols using 2-benzyloxy-1-methylpyridinium trifluoromethanesulfanonate: Methyl (R)-(-)-3-benzyloxy-2-methyl propanoate". Organic Syntheses. 84: 295. doi:10.15227/orgsyn.084.0295.
- ↑ Neeman, M.; Johnson, William S. (1961). "Cholestanyl methyl ether". Organic Syntheses. 41: 9. doi:10.15227/orgsyn.041.0009.
- ↑ Purdie, T.; Irvine, J. C. (1903). "C.?The alkylation of sugars". Journal of the Chemical Society, Transactions. 83: 1021. doi:10.1039/CT9038301021.
- ↑ Icke, Roland N.; Wisegarver, Burnett B.; Alles, Gordon A. (1945). "β-Phenylethyldimethylamine". Organic Syntheses. 25: 89. doi:10.15227/orgsyn.025.0089.
- ↑ Shioiri, Takayuki; Aoyama, Toyohiko; Snowden, Timothy (2001). "Trimethylsilyldiazomethane". e-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rt298.pub2.
- ↑ Lipsky, Sharon D.; Hall, Stan S. (1976). "Aromatic Hydrocarbons from aromatic ketones and aldehydes: 1,1-Diphenylethane". Organic Syntheses. 55: 7. doi:10.15227/orgsyn.055.0007.
- ↑ Grummitt, Oliver; Becker, Ernest I. (1950). "trans-1-Phenyl-1,3-butadiene". Organic Syntheses. 30: 75. doi:10.15227/orgsyn.030.0075.
- ↑ Negishi, Ei-ichi; Matsushita, Hajime (1984). "Palladium-Catalyzed Synthesis of 1,4-Dienes by Allylation of Alkenyalane: α-Farnesene". Organic Syntheses. 62: 31. doi:10.15227/orgsyn.062.0031.
- ↑ Wienken CJ, Baaske P, Duhr S, Braun D (2011). "Thermophoretic melting curves quantify the conformation and stability of RNA and DNA". Nucleic Acids Research. 39 (8): e52–e52. doi:10.1093/nar/gkr035. PMC 3082908. PMID 21297115.
External links
| Wikimedia Commons has media related to Methylation. |
| Look up methylation in Wiktionary, the free dictionary. |
- deltaMasses Detection of Methylations after Mass Spectrometry