L-selectin
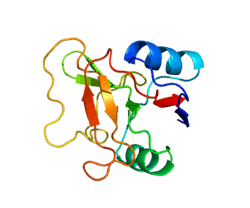
L-selectin, also known as CD62L, is a cell adhesion molecule found on lymphocytes and the preimplantation embryo. It belongs to the selectin family of proteins, which recognize sialylated carbohydrate groups. It is cleaved by ADAM17.
SELL is a cell surface component that is a member of a family of adhesion/homing receptors that play important roles in lymphocyte-endothelial cell interactions. The molecule is composed of multiple domains: one homologous to lectins, one to epidermal growth factor, and two to the consensus repeat units found in C3/C4-binding proteins.[5]
Ligands
- GlyCAM-1, found in the high endothelial venules of the lymph nodes.
- CD34, found on endothelial cells.
- MadCAM-1, found on endothelial cells of gut-associated lymphoid tissue.
- PSGL-1, binds with low affinity.
Function
L-selectin acts as a "homing receptor" for lymphocytes to enter secondary lymphoid tissues via high endothelial venules. Ligands present on endothelial cells will bind to lymphocytes expressing L-selectin, slowing lymphocyte trafficking through the blood, and facilitating entry into a secondary lymphoid organ at that point.[6] The receptor is commonly found on the cell surfaces of T cells. Naive T-lymphocytes, which have not yet encountered their specific antigen, need to enter secondary lymph nodes to encounter their antigen. Central memory T-lymphocytes, which have encountered antigen, express L-selectin to localize in secondary lymphoid organs. Here they reside ready to proliferate upon re-encountering antigen. Effector memory T-lymphocytes do not express L-selectin, as they circulate in the periphery and have immediate effector functions upon encountering antigen.
High expression of L-selectin on human bone marrow progenitor cells is an early sign of cells becoming committed to lymphoid differentiation.[7]
L-selectin is also present on the surface of human embryo trophoblasts prior to implantation into the uterus. Similar to its function in lymphocytes, L-selectin acts as a receptor to facilitate adhesion of the embryo to the site of invasion on the surface epithelium of the uterine endometrium. The embryo secretes human chorionic gonadotropin (hCG), which downregulates anti-adhesion factor, MUC-1, located on the uterine epithelium at the site of invasion. Removal of MUC-1 exposes the oligosaccharide ligands of the uterine epithelium, thus allowing binding by the L-selectin receptor of the trophopblast cell, followed by embryo adhesion and invasion.[8]
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000188404 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000026581 - Ensembl, May 2017
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ "Entrez Gene: SELL selectin L (lymphocyte adhesion molecule 1)".
- ↑ Robbins SL, Cotran RS, Kumar V, Collins T (1998). Robbins Pathologic Basis of Disease. Philadelphia: W.B Saunders Company. ISBN 0-7216-7335-X.
- ↑ Kohn LA, Hao QL, Sasidharan R, Parekh C, Ge S, Zhu Y, Mikkola HK, Crooks GM (October 2012). "Lymphoid priming in human bone marrow begins before expression of CD10 with upregulation of L-selectin". Nat. Immunol. 13 (10): 963–71. doi:10.1038/ni.2405. PMC 3448017. PMID 22941246.
- ↑ James JL, Carter AM, Chamley LW (May 2012). "Human placentation from nidation to 5 weeks of gestation. Part I: What do we know about formative placental development following implantation?". Placenta. 33 (5): 327–34. doi:10.1016/j.placenta.2012.01.020. PMID 22374510.
External links
- L-Selectin at the US National Library of Medicine Medical Subject Headings (MeSH)
- Immunology at MCG 1/physresp
Further reading
- Ryan US, Worthington RE (1992). "Cell-cell contact mechanisms". Curr. Opin. Immunol. 4 (1): 33–7. doi:10.1016/0952-7915(92)90120-4. PMID 1375831.
- Nicholson IC (2003). "CD62L (L-selectin)". J. Biol. Regul. Homeost. Agents. 16 (2): 144–6. PMID 12144128.
- Ivetic A, Ridley AJ (2005). "The telling tail of L-selectin". Biochem. Soc. Trans. 32 (Pt 6): 1118–21. doi:10.1042/BST0321118. PMID 15506984.
- Lasky LA, Singer MS, Dowbenko D, et al. (1992). "An endothelial ligand for L-selectin is a novel mucin-like molecule". Cell. 69 (6): 927–38. doi:10.1016/0092-8674(92)90612-G. PMID 1376638.
- Ord DC, Ernst TJ, Zhou LJ, et al. (1990). "Structure of the gene encoding the human leukocyte adhesion molecule-1 (TQ1, Leu-8) of lymphocytes and neutrophils". J. Biol. Chem. 265 (14): 7760–7. PMID 1692315.
- Bevilacqua M, Butcher E, Furie B, et al. (1991). "Selectins: a family of adhesion receptors". Cell. 67 (2): 233. doi:10.1016/0092-8674(91)90174-W. PMID 1717161.
- Tedder TF, Isaacs CM, Ernst TJ, et al. (1989). "Isolation and chromosomal localization of cDNAs encoding a novel human lymphocyte cell surface molecule, LAM-1. Homology with the mouse lymphocyte homing receptor and other human adhesion proteins". J. Exp. Med. 170 (1): 123–33. doi:10.1084/jem.170.1.123. PMC 2189363. PMID 2473156.
- Camerini D, James SP, Stamenkovic I, Seed B (1989). "Leu-8/TQ1 is the human equivalent of the Mel-14 lymph node homing receptor". Nature. 342 (6245): 78–82. doi:10.1038/342078a0. PMID 2509939.
- Bowen BR, Nguyen T, Lasky LA (1989). "Characterization of a human homologue of the murine peripheral lymph node homing receptor". J. Cell Biol. 109 (1): 421–7. doi:10.1083/jcb.109.1.421. PMC 2115458. PMID 2663882.
- Siegelman MH, Weissman IL (1989). "Human homologue of mouse lymph node homing receptor: evolutionary conservation at tandem cell interaction domains". Proc. Natl. Acad. Sci. U.S.A. 86 (14): 5562–6. doi:10.1073/pnas.86.14.5562. PMC 336895. PMID 2664786.
- Bajorath J, Aruffo A (1995). "A template for generation and comparison of three-dimensional selectin models". Biochem. Biophys. Res. Commun. 216 (3): 1018–23. doi:10.1006/bbrc.1995.2722. PMID 7488174.
- Dianzani U, Bragardo M, Buonfiglio D, et al. (1995). "Modulation of CD4 lateral interaction with lymphocyte surface molecules induced by HIV-1 gp120". Eur. J. Immunol. 25 (5): 1306–11. doi:10.1002/eji.1830250526. PMID 7539755.
- Maruyama K, Sugano S (1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Brenner B, Gulbins E, Schlottmann K, et al. (1997). "L-selectin activates the Ras pathway via the tyrosine kinase p56lck". Proc. Natl. Acad. Sci. U.S.A. 93 (26): 15376–81. doi:10.1073/pnas.93.26.15376. PMC 26412. PMID 8986819.
- Zöllner O, Lenter MC, Blanks JE, et al. (1997). "L-selectin from human, but not from mouse neutrophils binds directly to E-selectin". J. Cell Biol. 136 (3): 707–16. doi:10.1083/jcb.136.3.707. PMC 2134294. PMID 9024699.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, et al. (1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Prakobphol A, Thomsson KA, Hansson GC, et al. (1998). "Human low-molecular-weight salivary mucin expresses the sialyl lewisx determinant and has L-selectin ligand activity". Biochemistry. 37 (14): 4916–27. doi:10.1021/bi972612a. PMID 9538010.
- Sassetti C, Tangemann K, Singer MS, et al. (1998). "Identification of podocalyxin-like protein as a high endothelial venule ligand for L-selectin: parallels to CD34". J. Exp. Med. 187 (12): 1965–75. doi:10.1084/jem.187.12.1965. PMC 2212365. PMID 9625756.
- Malhotra R, Ward M, Sim RB, Bird MI (1999). "Identification of human complement Factor H as a ligand for L-selectin". Biochem. J. 341 (1): 61–9. doi:10.1042/0264-6021:3410061. PMC 1220330. PMID 10377245.
- Bradley LM, Duncan DD, Tonkonogy S, Swain SL (1999). "CD4+ effector T cells in vivo: immunization results in a transient population of MEL-14-, CD45RB- helper cells that secretes interleukin 2 (IL-2), IL-3, IL-4, and interferon gamma". J. Exp. Med. 174 (3): 547–59. doi:10.1084/jem.174.3.547. PMC 2118927. PMID 1678774.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.