S1PR1
Sphingosine-1-phosphate receptor 1 (S1P receptor 1 or S1P1), also known as endothelial differentiation gene 1 (EDG1) is a protein that in humans is encoded by the S1PR1 gene. S1PR1 is a G-protein-coupled receptor which binds the bioactive signaling molecule sphingosine 1-phosphate (S1P). S1PR1 belongs to a sphingosine-1-phosphate receptor subfamily comprising five members (S1PR1-5).[5] S1PR1 was originally identified as an abundant transcript in endothelial cells[6] and it has an important role in regulating endothelial cell cytoskeletal structure, migration, capillary-like network formation and vascular maturation.[7][8] In addition, S1PR1 signaling is important in the regulation of lymphocyte maturation, migration and trafficking.[9][10]
Structure
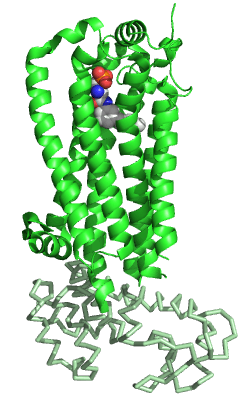
S1PR1 like the other members of the GPCR family is composed of seven-transmembrane helices arranged in a structurally conserved bundle.[5] As well as the other GPCRs, in the extracellular region S1PR1 is composed of three loops: ECL1 between helices II and III, ECL2 between helices IV and V and ECL3 between helices VI and VII. Compared to the other members of the family, S1PR1 has some specific features. The N-terminal of the protein folds as a helical cap above the top of the receptor and therefore it limits the access of the ligands to the amphipathic binding pocket. This marked amphipathicity is indeed in agreement with the zwitterionic nature of S1P. In addition, helices ECL1 and ECL2 pack tightly against the N-terminal helix, further occluding the access of the ligand from the extracellular space. S1P or S1P analogs are likely to reach the binding pocket from within the cell membrane and not from the extracellular space, may be through an opening between helices I and VII. Compared to the other GPCRs, this region is more open due to a different positioning of helices I and II toward helix III.[5] This occlusion of the ligand access space from the extracellular space could also explain the slow saturation of receptor binding in the presence of excess of ligand.[11]
Function
Like the other members of the GPCR family, S1PR1 senses its ligand from outside the cell and activates intracellular signal pathways that at last lead to cellular responses. The signal is transduced through the association of the receptor with different G proteins, which recruits a series of systems for downstream amplification of the signal.[12]
Immune system
S1PR1 activation is heavily involved in immune cell regulation and development. Sphingosine-1-phosphate receptor 1 is also involved in immune-modulation and directly involved in suppression of innate immune responses from T cells.[13] Depending on the G protein coupled with the S1PR1, diverse cellular effects are achieved: Gαi and Gαo modulate cellular survival, proliferation and motility; Gα12 and Gα13 modulate cytoskeletal remodeling and cell-shape changes and Gαq modulates several cellular effector functions.[12] All the intracellular functions occur via the interaction with Gαi and Gαo: these two proteins recruit other proteins for downstream amplification of the signal.[12] The main functions of S1P-S1PR1 system are as follows:
- The phosphatidylinositol 3-kinase (PI3K) and the lipid dependent protein kinase B (PKB) signaling pathway increases the survival of lymphocytes and other immune cells by inhibiting apoptosis.
- Phosphoinositide 3-kinase (PI3K) and the GTPase RAC are responsible of the lymphocytes migration and their interactions with other cells or with connective-tissue surfaces.[12] S1PR1-deficient thymocytes do not emigrate from the thymus, resulting in an increased numbers of mature thymocytes in the thymus and in medullary hyperplasia, and few S1PR1-deficient T cells can be detected in the blood, lymph nodes, spleen or non-lymphoid organs in these mouse models.[9][10] The proliferation of immune cells is due to S1P-mediated signals via the GTPase RAS and extracellular-signal regulated kinase (ERK). IV) The Phospholipase C (PLC)-induced increases in intracellular calcium levels allow the secretion of cytokines and other immune mediators.[12]
Vasculogenesis
S1PR1 is one of the main receptors responsible for vascular growth and development, at least during embryogenesis.[14] In vascular endothelial cells the binding of S1P to S1PR1 induces migration, proliferation, cell survival and morphogenesis into capillary-like structures.[15] Moreover, the binding of S1P to S1PR1 is implicated in the formation of cell-cell adherens junctions, therefore inhibiting paracellular permeability of solutes and macromolecules.[16][17] It was also shown in vivo that S1P synergizes with angiogenic factors such as FGF-2 and VEGF in inducing angiogenesis and vascular maturation through S1PR1.[17][18] showed that S1PR1-KO mice died during development due to a defect in vascular stabilization, suggesting that this receptor is essential for vascular development. In conclusion, several evidences confirm that S1P via S1PR1 is a potent regulator of vascular growth and development, at least during embryogenesis.[14]
Clinical significance
Cancer
S1PR1 is involved in the motility of cancer cells upon stimulation by S1P. The signal pathway involves RAC-CDC42 and correlates with ERK1 and ERK2 activation. The RAC-CDC42 pathway leads to cell migration, whereas the ERK pathway leads to proliferation and neovascularization[19][20] demonstrated that S1PR1 is strongly induced in endothelial cells during tumor angiogenesis and a siRNA against S1PR1 was able to inhibit angiogenesis and tumor growth. S1PR1 is also involved in other types of cancer: fibrosarcoma cells migrate upon activation of S1PR1 by S1P via RAC1–CDC42 dependent pathway)[21][22] and ovarian cancer cell invasion involves S1PR1 or S1PR3 and calcium mobilization.[23]
Multiple sclerosis
S1PR1 is involved in multiple sclerosis. Fingolimod, a drug which internalizes the receptor, is approved as a disease modifying agent in MS. There are other Sphingosine-1-phosphate receptor modulators. Van Doorn et al. (2010)[24] observed a strong increase in S1PR1 (and S1PR3) expression in hypertrophic astrocytes both in the active and inactive MS lesions from MS patients compared to the unaffected patients.
See also
References
- GRCh38: Ensembl release 89: ENSG00000170989 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000045092 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Hanson MA, Roth CB, Jo E, Griffith MT, Scott FL, Reinhart G, Desale H, Clemons B, Cahalan SM, Schuerer SC, Sanna MG, Han GW, Kuhn P, Rosen H, Stevens RC (February 2012). "Crystal structure of a lipid G protein-coupled receptor". Science. 335 (6070): 851–5. doi:10.1126/science.1215904. PMC 3338336. PMID 22344443.
- Hla T, Maciag T (June 1990). "An abundant transcript induced in differentiating human endothelial cells encodes a polypeptide with structural similarities to G-protein-coupled receptors". J. Biol. Chem. 265 (16): 9308–13. PMID 2160972.
- Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, Spiegel S, Hla T (March 1998). "Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1". Science. 279 (5356): 1552–5. doi:10.1126/science.279.5356.1552. PMID 9488656.
- Liu CH, Thangada S, Lee MJ, Van Brocklyn JR, Spiegel S, Hla T (April 1999). "Ligand-induced trafficking of the sphingosine-1-phosphate receptor EDG-1". Mol. Biol. Cell. 10 (4): 1179–90. doi:10.1091/mbc.10.4.1179. PMC 25247. PMID 10198065.
- Allende ML, Dreier JL, Mandala S, Proia RL (April 2004). "Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration". J. Biol. Chem. 279 (15): 15396–401. doi:10.1074/jbc.M314291200. PMID 14732704.
- Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG (January 2004). "Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1". Nature. 427 (6972): 355–60. doi:10.1038/nature02284. PMID 14737169.
- Rosen H, Gonzalez-Cabrera PJ, Sanna MG, Brown S (2009). "Sphingosine 1-phosphate receptor signaling". Annu. Rev. Biochem. 78: 743–68. doi:10.1146/annurev.biochem.78.072407.103733. PMID 19231986.
- Rosen H (September 2005). "Chemical approaches to the lysophospholipid receptors". Prostaglandins Other Lipid Mediat. 77 (1–4): 179–84. doi:10.1016/j.prostaglandins.2004.09.011. PMID 16099402.
- Sharma N, Akhade AS, Qadri A (2013). "Sphingosine-1-phosphate suppresses TLR-induced CXCL8 secretion from human T cells". J Leukoc Biol. 93 (4): 521–528. doi:10.1189/jlb.0712328. PMID 23345392.
- Chae SS, Paik JH, Allende ML, Proia RL, Hla T (April 2004). "Regulation of limb development by the sphingosine 1-phosphate receptor S1p1/EDG-1 occurs via the hypoxia/VEGF axis". Dev. Biol. 268 (2): 441–7. doi:10.1016/j.ydbio.2004.01.001. PMID 15063179.
- Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M, Volpi M, Sha'afi RI, Hla T (October 1999). "Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate". Cell. 99 (3): 301–12. doi:10.1016/S0092-8674(00)81661-X. PMID 10555146.
- Sanchez T, Estrada-Hernandez T, Paik JH, Wu MT, Venkataraman K, Brinkmann V, Claffey K, Hla T (November 2003). "Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability". J. Biol. Chem. 278 (47): 47281–90. doi:10.1074/jbc.M306896200. PMID 12954648.
- Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D (September 2001). "Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement". J. Clin. Invest. 108 (5): 689–701. doi:10.1172/JCI12450. PMC 209379. PMID 11544274.
- Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, Rosenfeldt HM, Nava VE, Chae SS, Lee MJ, Liu CH, Hla T, Spiegel S, Proia RL (October 2000). "Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation". J. Clin. Invest. 106 (8): 951–61. doi:10.1172/JCI10905. PMC 314347. PMID 11032855.
- Pyne NJ, Pyne S (July 2010). "Sphingosine 1-phosphate and cancer" (PDF). Nat. Rev. Cancer. 10 (7): 489–503. doi:10.1038/nrc2875. PMID 20555359.
- Chae SS, Paik JH, Furneaux H, Hla T (October 2004). "Requirement for sphingosine 1-phosphate receptor-1 in tumor angiogenesis demonstrated by in vivo RNA interference". J. Clin. Invest. 114 (8): 1082–9. doi:10.1172/JCI22716. PMC 522258. PMID 15489955.
- Fisher KE, Pop A, Koh W, Anthis NJ, Saunders WB, Davis GE (2006). "Tumor cell invasion of collagen matrices requires coordinate lipid agonist-induced G-protein and membrane-type matrix metalloproteinase-1-dependent signaling". Mol. Cancer. 5: 69. doi:10.1186/1476-4598-5-69. PMC 1762019. PMID 17156449.
- Nyalendo C, Michaud M, Beaulieu E, Roghi C, Murphy G, Gingras D, Béliveau R (May 2007). "Src-dependent phosphorylation of membrane type I matrix metalloproteinase on cytoplasmic tyrosine 573: role in endothelial and tumor cell migration". J. Biol. Chem. 282 (21): 15690–9. doi:10.1074/jbc.M608045200. PMID 17389600.
- Park KS, Kim MK, Lee HY, Kim SD, Lee SY, Kim JM, Ryu SH, Bae YS (April 2007). "S1P stimulates chemotactic migration and invasion in OVCAR3 ovarian cancer cells". Biochem. Biophys. Res. Commun. 356 (1): 239–44. doi:10.1016/j.bbrc.2007.02.112. PMID 17349972.
- Van Doorn R, Van Horssen J, Verzijl D, Witte M, Ronken E, Van Het Hof B, Lakeman K, Dijkstra CD, Van Der Valk P, Reijerkerk A, Alewijnse AE, Peters SL, De Vries HE (September 2010). "Sphingosine 1-phosphate receptor 1 and 3 are upregulated in multiple sclerosis lesions". Glia. 58 (12): 1465–76. doi:10.1002/glia.21021. PMID 20648639.
- Salim K, Fenton T, Bacha J, Urien-Rodriguez H, Bonnert T, Skynner HA, Watts E, Kerby J, Heald A, Beer M, McAllister G, Guest PC (May 2002). "Oligomerization of G-protein-coupled receptors shown by selective co-immunoprecipitation". J. Biol. Chem. 277 (18): 15482–5. doi:10.1074/jbc.M201539200. PMID 11854302.
- Lee MJ, Evans M, Hla T (May 1996). "The inducible G protein-coupled receptor edg-1 signals via the G(i)/mitogen-activated protein kinase pathway". J. Biol. Chem. 271 (19): 11272–9. doi:10.1074/jbc.271.19.11272. PMID 8626678.
External links
- "Lysophospholipid Receptors: S1P1". IUPHAR Database of Receptors and Ion Channels. International Union of Basic and Clinical Pharmacology.
- Lysophospholipid+receptors at the US National Library of Medicine Medical Subject Headings (MeSH)
This article incorporates text from the United States National Library of Medicine, which is in the public domain.