Titanium(III) fluoride
 | |
 | |
| Names | |
|---|---|
| IUPAC name
trifluorotitanium | |
| Other names
Titanium trifluoride, Titanous fluoride, titanium fluoride, titanium fluorideminpurplebrownpowder, trifluorotitanium | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.033.379 |
| EC Number | 236-732-4 |
PubChem CID |
|
| |
| |
| Properties | |
| TiF3 | |
| Molar mass | 104.862 g/mol |
| Appearance | violet to purple-red powder |
| Density | 3.4 g/cm3 |
| Melting point | 1,200 °C (2,190 °F; 1,470 K) |
| Boiling point | 1,400 °C (2,550 °F; 1,670 K) |
| decomposes | |
| +1300·10−6 cm3/mol | |
| Structure | |
| Rhombohedral, hR24 | |
| R-3c, No. 167 | |
| Hazards | |
EU classification (DSD) (outdated) |
not listed |
| Related compounds | |
Related compounds |
Titanium difluoride Titanium trichloride Titanium tribromide Titanium triiodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
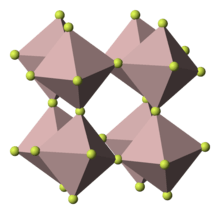
Titanium(III) fluoride (TiF3) is a inorganic compound with the formula TiF3. It is a violet solid. It adopts a perovskite-like structure such that each Ti center has octahedral coordination geometry and each fluoride ligand is doubly bridging.[1]
It can be obtained by the reaction of titanium(III) oxide with hydrofluoric acid. This reaction reverses if the TiF3 is dissolved in water.
References
- ↑ H. Sowa; H. Ahsbahs (1998). "Pressure-Induced Octahedron Strain in VF3-Type Compounds". Acta Crystallogr. B54: 578–584. doi:10.1107/S0108768198001207.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.