Lead titanate
 | |
| Names | |
|---|---|
| Other names
Lead(II) titanate Lead titanium oxide Lead(II) titanium oxide | |
| Identifiers | |
| ECHA InfoCard | 100.031.841 |
PubChem CID |
|
| Properties | |
| PbTiO3 | |
| Molar mass | 303.09 g/mol |
| Appearance | Yellow powder |
| Density | 7.52 g/cm3 |
| Insoluble | |
| Hazards | |
| Main hazards | Toxic (T) Dangerous for the environment (N) May damage fertility or unborn child |
| R-phrases (outdated) | R20/22, R33, R50/53, R61, R62[1] |
| S-phrases (outdated) | S45, S53, S60, S61[1] |
| NFPA 704 | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
12000 mg/kg (rat) |
| Related compounds | |
Other anions |
Lead dioxide Lead acetate |
Other cations |
Caesium titanate Iron(II) titanate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Lead(II) titanate is an inorganic compound with the chemical formula PbTiO3. It is the lead salt of titanic acid. Lead(II) titanate is a yellow powder that is insoluble in water.
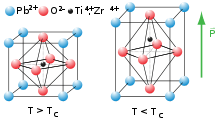
At high temperatures, lead titanate adopts a cubic perovskite structure. At 760 K,[2] the material undergoes a second order phase transition to a tetragonal perovskite structure which exhibits ferroelectricity. Lead titanate is one of the end members of the lead zirconate titanate (Pb[ZrxTi1-x]O3 0≤x≤1, PZT) system, which is technologically one of the most important ferroelectric and piezoelectric ceramics; PbTiO3 has a high ratio of k33 to kp with a high kt.
Toxicity
Lead titanate is toxic, like other lead compounds. It irritates skin, mucous membranes and eyes. It may also cause harm to unborn babies and might have effects on fertility.[5]
Solubility in water
The solubility of hydrothermally-synthesized perovskite-phase PbTiO3 in water was experimentally determined at 25 and 80 °C to depend on pH and vary from 4.9x10−4 mol/kg at pH≈3, to 1.9x10−4 mol/kg at pH≈7.7, to "undetectable" (<3.2x10−7 mol/kg) in the range 10<pH<11. At still higher pH values, the solubility increased again. The solubility was apparently incongruent and was quantified as the analytical concentration of Pb.[6]
References
- 1 2 Alfa Aesar "Archived copy". Archived from the original on 2011-07-07. Retrieved 2010-09-12.
- ↑ Noheda, Cereceda, Iglesias, Lifante, Gonzalo, Chen and Wang, Phys. Rev. B 51, 16388 (1995)
- ↑ Radusinović, Dušan and Markov, Cvetko "Macedonite - lead titanate: a new mineral", American Mineralogist 56, 387-394 (1971), http://www.minsocam.org/ammin/AM56/AM56_387.pdf
- ↑ Burke, E.A.J. and Kieft, C. "Second occurrence of makedonite, PbTiO3, Långban, Sweden", Lithos 4, 101-104 (1971)
- ↑ http://www.alfa.com/content/msds/USA/35671.pdf
- ↑ Jooho Moon, Melanie L. Carasso, Henrik G. Krarup, Jeffrey A. Kerchner, "Particle-shape control and formation mechanisms of hydrothermally derived lead titanate", Journal of Materials Research, Vol. 14, No.3, March 1999.
