Cobalt(III) fluoride
 | |
| Names | |
|---|---|
| Other names
Cobalt trifluoride Cobaltic fluoride Cobalt fluoride Cobaltic trifluoride | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.030.045 |
| EC Number | 233-062-4 |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| CoF3 | |
| Molar mass | 115.928 g/mol |
| Appearance | brown powder |
| Density | 3.88 g/cm3 |
| Melting point | 927 °C (1,701 °F; 1,200 K) |
| reacts | |
| +1900.0·10−6 cm3/mol | |
| Structure | |
| hexagonal | |
| Hazards | |
| NFPA 704 | |
| Related compounds | |
Other anions |
cobalt(III) oxide, cobalt(III) chloride |
Other cations |
iron(III) fluoride, rhodium(III) fluoride |
Related compounds |
cobalt(II) fluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Cobalt(III) fluoride is the inorganic compound with the formula CoF3. A dihydrate is also known. The anhydrous compound is a hygroscopic brown solid. It is used to synthesize organofluorine compounds.[1]
Preparation
CoF3 is prepared in the laboratory by treating CoCl2 with fluorine at 250 °C:[2]
- CoCl2 + 3/2 F2 → CoF3 + Cl2
In this redox reaction, Co2+ and Cl− are oxidized to Co3+ and Cl2, respectively, while F2 is reduced to F−. Cobalt(II) oxide (CoO) and cobalt(II) fluoride (CoF2) can also be converted to cobalt(III) fluoride using fluorine.
Structure and reactions
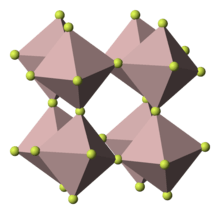
The compound crystallizes in the aluminium trifluoride motif. Each fluoride is a doubly bridging ligand. The cobalt centers are octahedral.
CoF3 decomposes upon contact with water to give oxygen:
- 4 CoF3 + 2 H2O → 4 HF + 4 CoF2 + O2
It reacts with fluoride salts to give the anion [CoF6]3−, which is also features high-spin, octahedral cobalt(III) center.
Applications
CoF3 is a powerful fluorinating agentUsed as slurry, CoF3 converts hydrocarbons to the perfluorocarbons:
- 2 CoF3 + R-H → 2 CoF2 + R-F + HF
CoF2 is the byproduct.
Such reactions are sometimes accompanied by rearrangements or other reactions.[1] The related reagent KCoF4 is more selective.[3]
Gaseous CoF3
In the gas phase, CoF3 is calculated to be planar in its ground state, and has a 3-fold rotation axis (symmetry notation D3h). The Cr3+ ion has a ground state of 3d6 5D. The fluoride ligands split this state into, in energy order, 5A', 5E", and 5E' states. The first energy difference is small and the 5E" state is subject to the Jahn-Teller Effect, so this effect needs to be considered to be sure of the ground state. The energy lowering is small and does not change the energy order.[4] This calculation was the first treatment of the Jahn-Teller Effect using calculated energy surfaces.
References
- 1 2 Coe, P. L. (2004). "Cobalt(III) Fluoride". Encyclopedia of Reagents for Organic Synthesis. J. Wiley. doi:10.1002/047084289X.rc185.
- ↑ Priest, H. F. "Anhydrous Metal Fluorides" Inorganic Syntheses McGraw-Hill: New York, 1950; Vol. 3, pages 171-183. doi:10.1002/9780470132340.ch47
- ↑ Coe, P. L. "Potassium Tetrafluorocobaltate(III)" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. doi:10.1002/047084289X.rp251.
- ↑ Yates, J. H.; Pitzer, R. M. (1979). "Molecular and Electronic Structure of Transition Metal Trifluorides". J. Chem. Phys. 70 (9): 4049–4055. doi:10.1063/1.438027.
External links
| Wikimedia Commons has media related to Cobalt(III) fluoride. |
- National Pollutant Inventory - Cobalt fact sheet
- National Pollutant Inventory - Fluoride and compounds fact sheet
