Caesium fluoride
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Caesium fluoride | |
| Other names
Cesium fluoride | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.033.156 |
PubChem CID |
|
| RTECS number | FK9650000 |
| |
| |
| Properties | |
| CsF | |
| Molar mass | 151.903 g/mol[1] |
| Appearance | white crystalline solid |
| Density | 4.64 g/cm3[1] |
| Melting point | 703 °C (1,297 °F; 976 K) [1] |
| Boiling point | 1,251 °C (2,284 °F; 1,524 K) |
| 573.0 g/100 mL (25 °C)[1] | |
| Solubility | Insoluble in acetone, diethyl ether, pyridine and ethanol 191 g/100 mL in methanol. |
| -44.5·10−6 cm3/mol[2] | |
Refractive index (nD) |
1.477 |
| Structure | |
| cubic, cF8 | |
| Fm3m, No. 225[3] | |
Lattice volume (V) |
0.2169 nm3[3] |
Formula units (Z) |
4 |
| Octahedral | |
| 7.9 D | |
| Thermochemistry | |
Heat capacity (C) |
51.1 J/mol·K[4] |
Std molar entropy (S |
92.8 J/mol·K[4] |
Std enthalpy of formation (ΔfH |
-553.5 kJ/mol[4] |
Gibbs free energy (ΔfG˚) |
-525.5 kJ/mol[4] |
| Hazards | |
| Main hazards | toxic |
| Safety data sheet | External MSDS |
| GHS pictograms |    |
| GHS signal word | Danger |
| H301, H311, H315, H318, H331, H361f | |
| NFPA 704 | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions |
Caesium chloride Caesium bromide Caesium iodide Caesium astatide |
Other cations |
Lithium fluoride Sodium fluoride Potassium fluoride Rubidium fluoride Francium fluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Caesium fluoride or cesium fluoride is an inorganic compound usually encountered as a hygroscopic white solid. It is used in organic synthesis as a source of the fluoride anion.
Synthesis and properties
Caesium fluoride can be prepared by the reaction of caesium hydroxide (CsOH) with hydrofluoric acid (HF). The resulting salt can then be purified by recrystallization. The reaction is shown below:
- CsOH(aq) + HF(aq) → CsF(aq) + H2O(l)
Another way to make caesium fluoride is to react caesium carbonate (Cs2CO3) with hydrofluoric acid. The resulting salt can then be purified by recrystallization. The reaction is shown below:
- Cs2CO3(aq) + 2 HF(aq) → 2 CsF(aq) + H2O(l) + CO2(g)
In addition, elemental fluorine and caesium can be used to form caesium fluoride as well, but doing so is very impractical because of the expense.[6] While this is not a normal route of preparation, caesium metal reacts vigorously with all the halogens to form caesium halides. Thus, it burns with fluorine gas, F2, to form caesium fluoride, CsF according to the following reaction:
- 2 Cs(s) + F2(g) → 2 CsF(s)
CsF is more soluble than sodium fluoride or potassium fluoride. It is available in anhydrous form, and if water has been absorbed it is easy to dry by heating at 100 °C for two hours in vacuo.[7] CsF reaches a vapor pressure of 1 kilopascal at 825 °C, 10 kPa at 999 °C, and 100 kPa at 1249 °C.[8]
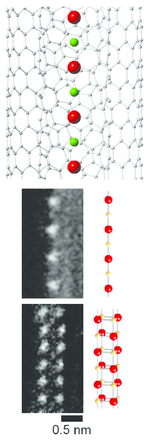
CsF chains with a thickness as small as one or two atoms can be grown inside carbon nanotubes.[5]
Structure
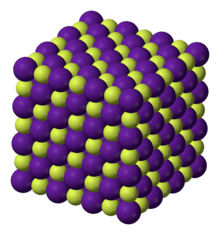
Caesium fluoride has the halite structure, which means that the Cs+ and F− pack in a cubic closest packed array as do Na+ and Cl− in sodium chloride.[3]
Applications in organic synthesis
Being highly dissociated it is a more reactive source of fluoride than related salts. CsF is less hygroscopic alternative to tetra-n-butylammonium fluoride (TBAF) and TAS-fluoride (TASF) when anhydrous "naked" fluoride ion is needed.
As a base
As with other soluble fluorides, CsF is moderately basic, because HF is a weak acid. The low nucleophilicity of fluoride means it can be a useful base in organic chemistry.[9] CsF gives higher yields in Knoevenagel condensation reactions than KF or NaF.[10]
Formation of C-F bonds
Caesium fluoride is also a popular source of fluoride in organofluorine chemistry. For example, CsF reacts with hexafluoroacetone to form a caesium perfluoroalkoxide salt, which is stable up to 60 °C, unlike the corresponding sodium or potassium salt. It will convert electron-deficient aryl chlorides to aryl fluorides (halex reaction).[11]
Deprotection agent
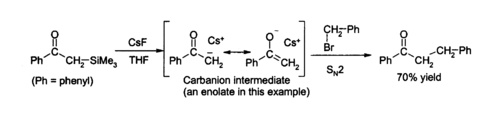
Due to the strength of the Si–F bond, fluoride ion is useful for desilylation reactions (removal of Si groups) in organic chemistry; caesium fluoride is an excellent source of anhydrous fluoride for such reactions. Removal of silicon groups (desilylation) is a major application for CsF in the laboratory, as its anhydrous nature allows clean formation of water-sensitive intermediates. Solutions of caesium fluoride in THF or DMF attack a wide variety of organosilicon compounds to produce an organosilicon fluoride and a carbanion, which can then react with electrophiles, for example:[10]
Desilylation is also useful for the removal of silyl protecting groups.[12]
Other uses
Single crystals of the salt are transparent into the deep infrared. For this reason it is sometimes used as the windows of cells used for infrared spectroscopy.
Precautions
Like other soluble fluorides, CsF is moderately toxic.[13] Contact with acid should be avoided, as this forms highly toxic/corrosive hydrofluoric acid. The caesium ion (Cs+) and caesium chloride are generally not considered toxic.[14]
References
- 1 2 3 4 Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 4.57. ISBN 1439855110.
- ↑ Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 4.132. ISBN 1439855110.
- 1 2 3 4 Davey, Wheeler P. (1923). "Precision Measurements of Crystals of the Alkali Halides". Physical Review. 21 (2): 143. doi:10.1103/PhysRev.21.143.
- 1 2 3 4 Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 5.10. ISBN 1439855110.
- 1 2 Senga, Ryosuke; Suenaga, Kazu (2015). "Single-atom electron energy loss spectroscopy of light elements". Nature Communications. 6: 7943. doi:10.1038/ncomms8943. PMC 4532884. PMID 26228378. (Supplementary information)
- ↑ Reacting Fluorine with Caesium. Youtube
- ↑ Friestad, G. K.; Branchaud, B. P. (1999). Reich, H. J.; Rigby, J. H., eds. Handbook of Reagents for Organic Synthesis: Acidic and Basic Reagents. New York: Wiley. pp. 99–103. ISBN 978-0-471-97925-8.
- ↑ Lide, D. R., ed. (2005). "Vapor Pressure". CRC Handbook of Chemistry and Physics (PDF) (86th ed.). Boca Raton (FL): CRC Press. p. 6.63. ISBN 0-8493-0486-5.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. pp. 82–83. ISBN 0-08-022057-6.
- 1 2 Fiorenza, M; Mordini, A; Papaleo, S; Pastorelli, S; Ricci, A (1985). "Fluoride ion induced reactions of organosilanes: the preparation of mono and dicarbonyl compounds from β-ketosilanes". Tetrahedron Letters. 26 (6): 787–788. doi:10.1016/S0040-4039(00)89137-6.
- ↑ Evans, F. W.; Litt, M. H.; Weidler-Kubanek, A. M.; Avonda, F. P. (1968). "Formation of adducts between fluorinated ketones and metal fluorides". Journal of Organic Chemistry. 33 (5): 1837–1839. doi:10.1021/jo01269a028.
- ↑ Smith, Adam P.; Lamba, Jaydeep J. S.; Fraser, Cassandra L. (2002). "Efficient Synthesis of Halomethyl-2,2'-bipyridines: 4,4'-Bis(chloromethyl)-2,2'-bipyridine". Organic Syntheses. 78: 82. ; Collective Volume, 10, p. 107
- ↑ MSDS Listing for cesium fluoride. www.hazard.com. MSDS Date: April 27, 1993. Retrieved on September 7, 2007.
- ↑ "MSDS Listing for cesium chloride." www.jtbaker.com. MSDS Date: January 16, 2006. Retrieved on September 7, 2007.
External links
| Wikimedia Commons has media related to Caesium fluoride. |