Prednisone
 | |
 | |
| Clinical data | |
|---|---|
| Synonyms | Deltasone, Liquid Pred, Orasone, Adasone, Deltacortisone, Prednisonum |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601102 |
| Pregnancy category | |
| Routes of administration | Oral, Nasal, Rectal, Injection, IV |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 70% |
| Metabolism | prednisolone (liver) |
| Elimination half-life | 3 to 4 hours in adults. 1 to 2 hours in children<[1] |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard |
100.000.147 |
| Chemical and physical data | |
| Formula | C21H26O5 |
| Molar mass | 358.428 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Prednisone is a synthetic glucocorticoid drug that is mostly used to suppress the immune system. It is used to treat certain inflammatory diseases (such as moderate allergic reactions), some autoimmune diseases, and (at higher doses) certain types of cancer. It is usually taken by mouth in the form of tablets.[2]
Common side effects include weight gain, high blood pressure, high blood sugar, mood changes, blurred vision, dizziness, and headache. Long-term use can lead to cataracts, bone loss, thrush, easy bruising and muscle weakness. It is generally considered safe to use during pregnancy.[3]:1219–1222
It is a prodrug to prednisolone, which mediates its glucocorticoid effects.[2][4] It works by binding to the glucocorticoid receptor, activating it and triggering changes in gene expression, that suppress the immune system and produce changes to metabolism.[3]:1215–1222
Medical uses
Prednisone is used for many different autoimmune diseases and inflammatory conditions, including: asthma, COPD, CIDP, rheumatic disorders, allergic disorders, ulcerative colitis and Crohn's disease, adrenocortical insufficiency, hypercalcemia due to cancer, thyroiditis, laryngitis, severe tuberculosis, urticaria (hives), lipid pneumonitis, pericarditis, multiple sclerosis, nephrotic syndrome, sarcoidosis, to relieve the effects of shingles, lupus, myasthenia gravis, poison oak exposure, Ménière's disease, autoimmune hepatitis, giant-cell arteritis, the Herxheimer reaction that is common during the treatment of syphilis, Duchenne muscular dystrophy, uveitis, and as part of a drug regimen to prevent rejection after organ transplant.[5][6][7]
Prednisone has also been used in the treatment of migraine headaches and cluster headaches and for severe aphthous ulcer. Prednisone is used as an antitumor drug.[8] It is important in the treatment of acute lymphoblastic leukemia, non-Hodgkin lymphomas, Hodgkin's lymphoma, multiple myeloma, and other hormone-sensitive tumors, in combination with other anticancer drugs.
Prednisone can be used in the treatment of decompensated heart failure to increase renal responsiveness to diuretics, especially in heart failure patients with refractory diuretic resistance with large dose of loop diuretics.[9][10][11][12][13][14] In terms of the mechanism of action for this purpose: prednisone, a glucocorticoid, can improve renal responsiveness to atrial natriuretic peptide by increasing the density of natriuretic peptide receptor type A in the renal inner medullary collecting duct, inducing a potent diuresis.[15]
Side effects
Short-term side effects, as with all glucocorticoids, include high blood glucose levels (especially in patients with diabetes mellitus or on other medications that increase blood glucose, such as tacrolimus) and mineralocorticoid effects such as fluid retention.[16] The mineralocorticoid effects of prednisone are minor, which is why it is not used in the management of adrenal insufficiency, unless a more potent mineralocorticoid is administered concomitantly.
It can also cause depression or depressive symptoms and anxiety in some individuals.[17][18]
Long-term side effects include Cushing's syndrome, steroid dementia syndrome,[19] truncal weight gain, osteoporosis, glaucoma and cataracts, diabetes mellitus type 2, and depression upon dose reduction or cessation.[20]
Major
- Increased blood sugar for individuals with diabetes
- Difficulty controlling emotion
- Difficulty in maintaining train of thought
- Weight gain
- Immunosuppression
- Severe facial swelling
- Depression, mania, psychosis, or other psychiatric symptoms
- Unusual fatigue or weakness
- Mental confusion / indecisiveness
- Memory and attention dysfunction (Steroid dementia syndrome)
- Muscle atrophy[21]
- Blurred vision
- Abdominal pain
- Peptic ulcer
- Painful hips or shoulders
- Steroid-induced osteoporosis
- Stretch marks
- Osteonecrosis – same as avascular necrosis
- Insomnia
- Severe joint pain
- Cataracts or glaucoma
- Anxiety
- Black stool
- Stomach pain or bloating
- Severe swelling
- Mouth sores or dry mouth
- Avascular necrosis
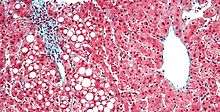
- Hepatic steatosis
Minor
- Nervousness
- Acne
- Skin rash
- Appetite gain
- Hyperactivity
- Increased thirst
- Frequent urination
- Diarrhea
- Reduced intestinal flora
- Leg pain/cramps
- Sensitive teeth
- Headache
- Induced vomiting
Dependency
Adrenal suppression will begin to occur if prednisone is taken for longer than seven days. Eventually, this may cause the body to temporarily lose the ability to manufacture natural corticosteroids (especially cortisol), which results in dependence on prednisone. For this reason, prednisone should not be abruptly stopped if taken for more than seven days; instead, the dosage should be gradually reduced. This weaning process may be over a few days if the course of prednisone was short, but may take weeks or months[22] if the patient had been on long-term treatment. Abrupt withdrawal may lead to an Addison crisis. For those on chronic therapy, alternate-day dosing may preserve adrenal function and thereby reduce side effects.[23]
Glucocorticoids act to inhibit feedback of both the hypothalamus, decreasing corticotropin-releasing hormone [CRH], and corticotrophs in the anterior pituitary gland, decreasing the amount of adrenocorticotropic hormone [ACTH]. For this reason, glucocorticoid analogue drugs such as prednisone down-regulate the natural synthesis of glucocorticoids. This mechanism leads to dependence in a short time and can be dangerous if medications are withdrawn too quickly. The body must have time to begin synthesis of CRH and ACTH and for the adrenal glands to begin functioning normally again.
Withdrawal
The magnitude and speed of dose reduction in corticosteroid withdrawal should be determined on a case-by-case basis, taking into consideration the underlying condition being treated, and individual patient factors such as the likelihood of relapse and the duration of corticosteroid treatment. Gradual withdrawal of systemic corticosteroids should be considered in those whose disease is unlikely to relapse and have:
- received more than 40 mg prednisone (or equivalent) daily for more than 1 week
- been given repeat doses in the evening;
- received more than 3 weeks' treatment
- recently received repeated courses (particularly if taken for longer than 3 weeks)
- taken a short course within 1 year of stopping long-term therapy
- other possible causes of adrenal suppression
Systemic corticosteroids may be stopped abruptly in those whose disease is unlikely to relapse and who have received treatment for 3 weeks or less and who are not included in the patient groups described above.
During corticosteroid withdrawal, the dose may be reduced rapidly down to physiological doses (equivalent to prednisolone 7.5 mg daily) and then reduced more slowly. Assessment of the disease may be needed during withdrawal to ensure that relapse does not occur.[24]
Pharmacology
Prednisone has no substantial biological effects until converted via hepatic metabolism to prednisolone.[25]
Industry
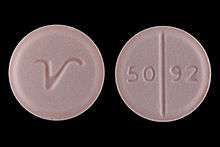
The pharmaceutical industry uses prednisone tablets for the calibration of dissolution testing equipment according to the United States Pharmacopeia (USP).
Chemistry
Prednisone is a synthetic pregnane corticosteroid and derivative of cortisone and is also known as δ1-cortisone or 1,2-dehydrocortisone or as 17α,21-dihydroxypregna-1,4-diene-3,11,20-trione.[26][27]
History
The first isolation and structure identifications of prednisone and prednisolone were done in 1950 by Arthur Nobile.[28][29][30] The first commercially feasible synthesis of prednisone was carried out in 1955 in the laboratories of Schering Corporation, which later became Schering-Plough Corporation, by Arthur Nobile and coworkers.[31] They discovered that cortisone could be microbiologically oxidized to prednisone by the bacterium Corynebacterium simplex. The same process was used to prepare prednisolone from hydrocortisone.[32]
The enhanced adrenocorticoid activity of these compounds over cortisone and hydrocortisone was demonstrated in mice.[32]
Prednisone and prednisolone were introduced in 1955 by Schering and Upjohn, under the brand names Meticorten[33] and Delta-Cortef,[34] respectively. These prescription medicines are now available from a number of manufacturers as generic drugs.
See also
References
- ↑ Pickup ME (1979). "Clinical pharmacokinetics of prednisone and prednisolone". Clinical Pharmacokinetics. 4 (2): 111–28. doi:10.2165/00003088-197904020-00004. PMID 378499.
- 1 2 "Product Information Panafcort® (prednisone) Panafcortelone® (prednisolone)" (PDF). TGA eBusiness Services. St Leonards, Australia: Aspen Pharmacare Australia Pty Ltd. 11 July 2017. p. 1–2. Retrieved 30 June 2018.
- 1 2 Brunton LL, Chabner B, Knollman B (2011). Goodman & Gilman's pharmacological basis of therapeutics (12th ed.). New York, USA: McGraw-Hill. ISBN 978-0-07-162442-8.
- ↑ Buttgereit F, Gibofsky A (June 2013). "Delayed-release prednisone - a new approach to an old therapy". Expert Opinion on Pharmacotherapy. 14 (8): 1097–106. doi:10.1517/14656566.2013.782001. PMID 23594208.
- ↑ Autoimmune Hepatitis~treatment at eMedicine
- ↑ "Corticosteroids". livertox.nlm.nih.gov. Retrieved 2017-04-07.
- ↑ "Prednisone". The American Society of Health-System Pharmacists. Retrieved 3 April 2011.
- ↑ "Antineoplastic Agents, Hormonal". Medical Subject Headings. U.S. National Library of Medicine. 2009. Retrieved 11 November 2010.
- ↑ Riemer AD (April 1958). "Application of the newer corticosteroids to augment diuresis in congestive heart failure". The American Journal of Cardiology. 1 (4): 488–96. doi:10.1016/0002-9149(58)90120-6. PMID 13520608.
- ↑ Newman DA (February 1959). "Reversal of intractable cardiac edema with prednisone". New York State Journal of Medicine. 59 (4): 625–33. PMID 13632954.
- ↑ Zhang H, Liu C, Ji Z, Liu G, Zhao Q, Ao YG, Wang L, Deng B, Zhen Y, Tian L, Ji L, Liu K (September 2008). "Prednisone adding to usual care treatment for refractory decompensated congestive heart failure". International Heart Journal. 49 (5): 587–95. doi:10.1536/ihj.49.587. PMID 18971570.
- ↑ Liu C, Liu G, Zhou C, Ji Z, Zhen Y, Liu K (September 2007). "Potent diuretic effects of prednisone in heart failure patients with refractory diuretic resistance" (Full text). The Canadian Journal of Cardiology. 23 (11): 865–8. doi:10.1016/s0828-282x(07)70840-1. PMC 2651362. PMID 17876376.
- ↑ Liu C, Chen H, Zhou C, Ji Z, Liu G, Gao Y, Tian L, Yao L, Zheng Y, Zhao Q, Liu K (October 2006). "Potent potentiating diuretic effects of prednisone in congestive heart failure". Journal of Cardiovascular Pharmacology. 48 (4): 173–6. doi:10.1097/01.fjc.0000245242.57088.5b. PMID 17086096.
- ↑ Massari F, Mastropasqua F, Iacoviello M, Nuzzolese V, Torres D, Parrinello G (March 2012). "The glucocorticoid in acute decompensated heart failure: Dr Jekyll or Mr Hyde?". The American Journal of Emergency Medicine. 30 (3): 517.e5–10. doi:10.1016/j.ajem.2011.01.023. PMID 21406321.
- ↑ Liu C, Chen Y, Kang Y, Ni Z, Xiu H, Guan J, Liu K (October 2011). "Glucocorticoids improve renal responsiveness to atrial natriuretic peptide by up-regulating natriuretic peptide receptor-A expression in the renal inner medullary collecting duct in decompensated heart failure". The Journal of Pharmacology and Experimental Therapeutics. 339 (1): 203–9. doi:10.1124/jpet.111.184796. PMID 21737535.
- 1 2 3 "Prednisone and other corticosteroids: Balance the risks and benefits - Mayo Clinic". Mayo Clinic. Retrieved 2017-04-07.
- ↑ "Prednisone Information". Drugs.com.
- ↑ "Prednisone". MedlinePlus Drug Information.
- ↑ Wolkowitz OM, Lupien SJ, Bigler ED (June 2007). "The "steroid dementia syndrome": a possible model of human glucocorticoid neurotoxicity". Neurocase. 13 (3): 189–200. doi:10.1080/13554790701475468. PMID 17786779.
- ↑ "Steroids". Australian Department of Health & Human Services. April 2016. Retrieved 2018-06-14.
- ↑ Schakman O, Gilson H, Kalista S, Thissen JP (November 2009). "Mechanisms of muscle atrophy induced by glucocorticoids". Hormone Research. 72 Suppl 1: 36–41. doi:10.1159/000229762. PMID 19940494.
- ↑ "Steroid Drug Withdrawal Symptoms, Treatment & Prognosis". MedicineNet. Retrieved 2018-06-14.
- ↑ "Therapeutic and Adverse Effects of Glucocorticoids". Bello CS, Garrett SD. U.S. Pharmacist Continuing Education Program no. 430-000-99-028-H01, August 1999. Archived from the original on 11 July 2008.
- ↑ Iliopoulou A, Abbas A, Murray R (2013-05-19). "How to manage withdrawal of glucocorticoid therapy". Prescriber. 24 (10): 23–29. doi:10.1002/psb.1060.
- ↑ Medline drug information for prednisone
- ↑ J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 1013–. ISBN 978-1-4757-2085-3.
- ↑ Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 871–. ISBN 978-3-88763-075-1.
- ↑ Wainwright M (1998). "The secret of success: Arthur Nobile's discovery of the steroids prednisone and prednisolone in the 1950s revolutionised the treatment of arthritis". Chemistry in Britain. 34 (1): 46. OCLC 106716069.
- ↑ "Inventor Profile: Arthur Nobile". National Inventors Hall of Fame. Archived from the original on 12 June 2012.
- ↑ "Arthur Nobile". New Jersey Inventors Hall of Fame. Archived from the original on 1 September 2011.
- ↑ Merck Index (14th ed.). Merck & Co. Inc. 2006. p. 1327. ISBN 978-0-911910-00-1.
- 1 2 Herzog HL, Nobile A, Tolksdorf S, Charney W, Hershberg EB, Perlman PL, Pechet MM (February 1955). "New antiarthritic steroids". Science. 121 (3136): 176. doi:10.1126/science.121.3136.176. PMID 13225767.
- ↑ "New Drug Application (NDA): 009766". FDA Approved Drug Products. U.S. Food and Drug Administration.
- ↑ "New Drug Application (NDA): 009987". FDA Approved Drug Products. U.S. Food and Drug Administration.
Further reading
- Tsang SY, Garovoy MR, Benet LZ (1985). "Immunosuppressive activity of prednisone and prednisolone and their metabolic interconversion in the mixed lymphocyte reaction". International Journal of Immunopharmacology. 7 (5): 731–7. doi:10.1016/0192-0561(85)90159-6. PMID 2931388.
External links
- Kelly, Janis (July 2000). "Prednisone-related growth impairment persists in children with CF". Respiratory Reviews. 5 (7). Archived from the original on 4 January 2013.
- National Inventors Hall of Fame induction of Arthur Nobile
- U.S. National Library of Medicine: Drug Information Portal – Prednisone
- The National Center for Biotechnology Information: Prednisone