Allylglycine
 | |
| Names | |
|---|---|
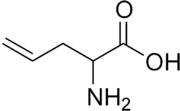
| IUPAC name
2-Aminopent-4-enoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.028.809 |
PubChem CID |
|
| |
| |
| Properties | |
| C5H9NO2 | |
| Molar mass | 115.13 g/mol |
| Appearance | white crystalline powder |
| Density | 1.098 g/mL |
| Melting point | 265 °C (509 °F; 538 K) |
| Boiling point | 231 °C (448 °F; 504 K) |
| Hazards | |
| Main hazards | Convulsant |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
147-195 mg/kg (mice)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Allylglycine is a glycine derivative. It is an inhibitor of glutamate decarboxylase.[2] Inhibition of glutamate decarboxylase blocks GABA biosynthesis, leading to lower levels of the neurotransmitter.[3] Allylglycine is known to induce seizures in animals studies, presumably due to this GDC-inhibiting activity.[4]
References
- ↑ Piepho, R. W; Friedman, A. H (1977). "CHRONOPHARMACOLOGY OF STRYCHNINE AND ALLYLGLYCINE IN THE MOUSE". Clinical and Experimental Pharmacology and Physiology. 4 (3): 263. doi:10.1111/j.1440-1681.1977.tb02623.x. PMID 891041.
- ↑ Abshire VM, Hankins KD, Roehr KE, DiMicco JA (November 1988). "Injection of L-allylglycine into the posterior hypothalamus in rats causes decreases in local GABA which correlate with increases in heart rate". Neuropharmacology. 27 (11): 1171–7. doi:10.1016/0028-3908(88)90013-5. PMID 3205383.
- ↑ Sajdyk T, Johnson P, Fitz S, Shekhar A (August 2008). "Chronic inhibition of GABA synthesis in the bed nucleus of the stria terminalis elicits anxiety-like behavior". J. Psychopharmacol. (Oxford). 22 (6): 633–41. doi:10.1177/0269881107082902. PMC 3065212. PMID 18308797.
- ↑ Thomas J, Yang YC (June 1991). "Allylglycine induced seizures in male and female rats". Physiol. Behav. 49 (6): 1181–3. PMID 1654571.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.