Dentate gyrus
| Dentate gyrus | |
|---|---|
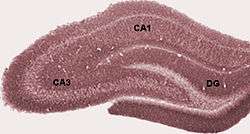 Diagram of hippocampal regions. DG: Dentate gyrus. | |
 Coronal section of brain immediately in front of pons. (Label for "Gyrus dentatus" is at bottom center.) | |
| Details | |
| Part of | Temporal lobe |
| Artery |
Posterior cerebral Anterior choroidal |
| Identifiers | |
| Latin | gyrus dentatus |
| MeSH | D018891 |
| NeuroNames | 179 |
| NeuroLex ID | birnlex_1178 |
| TA |
A14.1.09.237 A14.1.09.339 |
| FMA | 61922 |
| Anatomical terms of neuroanatomy | |
The dentate gyrus is part of a brain region known as the hippocampus (part of the hippocampal formation). The dentate gyrus is thought to contribute to the formation of new episodic memories,[1][2] the spontaneous exploration of novel environments,[2] and other functions.[3] It is notable as being one of a select few brain structures currently known to have significant rates of adult neurogenesis in many species of mammals, from rodents to primates[4] (other sites include the subventricular zone of the striatum[5] and cerebellum[6]). However, whether neurogenesis exists in the adult human dentate gyrus is currently a matter of debate.[7][8]
Structure
The dentate gyrus (DG) consists of three distinct layers: molecular, granular, and polymorphic, and participates in the 'hippocampal circuit' or trisynaptic loop.[9] The neurons of the granule cell layer are called granule cells and project axons called mossy fibers to synapse on the dendrites of CA3 pyramidal neurons. Granule cells of the DG receive excitatory input from the entorhinal cortex by way of the perforant pathway.[10] This input is primarily made up of signals from layer II of the entorhinal cortex, and the dentate gyrus receives no direct inputs from other cortical structures.[11] The perforant pathway is divided into the medial and lateral perforant paths, generated, respectively, at the medial and lateral portions of the entorhinal cortex. The medial perforant path synapses onto the proximal dendritic area of the granule cells, whereas the lateral perforant path does so onto their distal dendrites. Most lateral views of the dentate gyrus may appear to suggest a structure consisting of just one entity, but medial movement may provide evidence of the ventral and dorsal parts of the dentate gyrus.[12]
Development
The granule cells in the dentate gyrus are distinguished by their late time of formation during brain development. In rats, approximately 85% of the granule cells are generated after birth.[13] In humans, it is estimated that granule cells begin to be generated during gestation weeks 10.5 to 11, and continue being generated during the second and third trimesters, after birth and all the way into adulthood.[14][15] The germinal sources of granule cells and their migration pathways [16][17] have been studied during rat brain development. The oldest granule cells are generated in a specific region of the hippocampal neuroepithelium and migrate into the primordial dentate gyrus around embryonic days (E) 17/18, and then settle as the outermost cells in the forming granular layer. Next, dentate precursor cells move out of this same area of the hippocampal neuroepithelium and, retaining their mitotic capacity, invade the hilus (core) of the forming dentate gyrus. This dispersed germinal matrix is the source of granule cells from that point on. The newly generated granule cells accumulate under the older cells that began to settle in the granular layer. As more granule cells are produced, the layer thickens and the cells are stacked up according to age - the oldest being the most superficial and the youngest being deeper.[18] The granule cell precursors remain in a subgranular zone that becomes progressively thinner as the dentate gyrus grows, but these precursor cells are retained in adult rats. These sparsely scattered cells constantly generate granule cell neurons,[19][20] which add to the total population. There are a variety of other differences in the rat, monkey and human dentate gyrus. The granule cells only have apical dendrites in the rat. But in the monkey and human, many granule cells also have basal dendrites.[21]
Function
.jpg)
The dentate gyrus is thought to contribute to the formation of memories, and to play a role in depression.
Memory
The role of the hippocampus in learning and memory has been studied for many decades since early lesion studies. One of the most prominent early cases of anterograde amnesia (inability to form new memories) linking the hippocampus to memory formation was the case of Henry Molaison (anonymously known as Patient H.M. until his death in 2008).[23] His epilepsy was treated with surgical removal of his hippocampi (left and right hemispheres each have their own hippocampus) as well as some surrounding tissue. This targeted brain tissue removal left Mr. Molaison with an inability to form new memories, and the hippocampus has been thought critical to memory formation since that time.[23] It remains unclear how the hippocampus enables new memory formation, but one process, called long term potentiation (LTP), occurs in this brain region.[23] LTP involves long-lasting strengthening of synaptic connections after repeated stimulation.[10] While the dentate gyrus shows LTP, it is also one of the few regions of the adult mammalian brain where neurogenesis (i.e., the birth of new neurons) takes place. Some studies hypothesize that new memories could preferentially use newly formed dentate gyrus cells, providing a potential mechanism for distinguishing multiple instances of similar events or multiple visits to the same location.[24] This increased neurogenesis is associated with improved spatial memory in rodents, as seen through performance in a maze.[25]
Stress and depression
The dentate gyrus may also have a functional role in stress and depression. For instance, neurogenesis has been found to increase in response to chronic treatment with antidepressants.[26] The physiological effects of stress, often characterized by release of glucocorticoids such as cortisol, as well as activation of the sympathetic division of the autonomic nervous system, have been shown to inhibit the process of neurogenesis in primates.[27] Both endogenous and exogenous glucocorticoids are known to cause psychosis and depression,[28] implying that neurogenesis in the dentate gyrus may play an important role in modulating symptoms of stress and depression.[29]
Other
Some evidence suggests neurogenesis in the dentate gyrus increases in response to aerobic exercise.[30] Several experiments have shown neurogenesis (the development of nerve tissues) often increases in the dentate gyrus of adult rodents when they are exposed to an enriched environment.[31][32] The dentate gyrus is also known to serve as a pre-processing unit. When information enters, it is known to separate very similar information into distinct and unique details. This prepares the relevant data for storage in the hippocampal CA3 section.[33]
Spatial behavior
Studies have shown that after destroying about 90% of their dentate gyrus (dg) cells, rats had extreme difficulty in maneuvering through a maze they had been through, prior to the lesion being made. When being tested a number of times to see whether they could learn a maze, the results showed that the rats did not improve at all, indicating that their working memories were severely impaired. Rats had trouble with place strategies because they could not consolidate learned information about a maze into their working memory, and, thus, could not remember it when maneuvering through the same maze in a later trial. Every time a rat entered the maze, the rat behaved as if it was seeing the maze for the first time.[34]
Blood sugar
Studies by researchers at Columbia University Medical Center indicate poor glucose control can lead to deleterious effects on the dentate gyrus.[35]
References
- ↑ Amaral, David; Scharfman, Helen; Lavenex, Pierre (2007). The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies). Progress in Brain Research. 163. pp. 3–22, 788–790.
- 1 2 Saab BJ, Georgiou J, Nath A, Lee FJ, Wang M, Michalon A, Liu F, Mansuy IM, Roder JC (2009). "NCS-1 in the dentate gyrus promotes exploration, synaptic plasticity, and rapid acquisition of spatial memory". Neuron. 63 (5): 643–56. doi:10.1016/j.neuron.2009.08.014. PMID 19755107.
- ↑ Helen Scharfman, ed. (2007). The Dentate Gyrus: A comprehensive guide to structure, function, and clinical implications. Progress in Brain Research. 163. pp. 1–840.
- ↑ Cameron HA, McKay RD (2001). "Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus". J Comp Neurol. 435 (4): 406–17. doi:10.1002/cne.1040. PMID 11406822.
- ↑ Ernst, A; Alkass, K; Bernard, S; Salehpour, M; Perl, S; Tisdale, J; Possnert, G; Druid, H; Frisén, J (27 February 2014). "Neurogenesis in the striatum of the adult human brain". Cell. 156 (5): 1072–83. doi:10.1016/j.cell.2014.01.044. PMID 24561062.
- ↑ Ponti G, Peretto P, Bonfanti L (2008). "Genesis of neuronal and glial progenitors in the cerebellar cortex of peripuberal and adult rabbits". PLoS ONE. 3 (6): e2366. Bibcode:2008PLoSO...3.2366P. doi:10.1371/journal.pone.0002366. PMC 2396292. PMID 18523645.
- ↑ Sorrells, SF; Paredes, MF; Cebrian-Silla, A; Sandoval, K; Qi, D; Kelley, KW; James, D; Mayer, S; Chang, J; Auguste, KI; Chang, EF; Gutierrez, AJ; Kriegstein, AR; Mathern, GW; Oldham, MC; Huang, EJ; Garcia-Verdugo, JM; Yang, Z; Alvarez-Buylla, A (15 March 2018). "Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults". Nature. 555 (7696): 377–381. Bibcode:2018Natur.555..377S. doi:10.1038/nature25975. PMID 29513649.
- ↑ Boldrini, M; Fulmore, CA; Tartt, AN; Simeon, LR; Pavlova, I; Poposka, V; Rosoklija, GB; Stankov, A; Arango, V; Dwork, AJ; Hen, R; Mann, JJ (5 April 2018). "Human Hippocampal Neurogenesis Persists throughout Aging". Cell stem cell. 22 (4): 589–599.e5. doi:10.1016/j.stem.2018.03.015. PMID 29625071.
- ↑ A. Treves; A. Tashiro; M.P. Witter; E.I. Moser (2008). What is the mammalian dentate gyrus good for? (154th ed.). pp. 1155–1172.
- 1 2 Blumenfeld, Hal (2010). Neuroanatomy through clinical cases (2nd ed.). Sunderland, Mass.: Sinauer Associates. ISBN 978-0878936137.
- ↑ Nolte, John (2002). The Human Brain: An Introduction to Its Functional Neuroanatomy (fifth ed.). pp. 570–573.
- ↑ Rachel A. Dalley; Lydia L. Ng; Angela L. Guillozet-Bongaarts. "Dentate Gyrus". Nature Precedings. doi:10.1038/npre.2008.2095.1.
- ↑ Bayer, S.; Altman, J. (1974). "Hippocampal development in the rat: cytogenesis and morphogenesis examined with autoradiography and low-level X-irradiation". The Journal of Comparative Neurology. 158 (1): 55–79. doi:10.1002/cne.901580105. PMID 4430737.
- ↑ Bayer SA, Altman J (2008). The Human Brain During The Early First Trimester. 5 Atlas of Human Central Nervous System Development. Appendix, p. 497.
- ↑ Eriksson PS, Perfilieva E, Björk-Eriksson T, et al. (November 1998). "Neurogenesis in the adult human hippocampus". Nat. Med. 4 (11): 1313–7. doi:10.1038/3305. PMID 9809557.
- ↑ Altman, J.; Bayer, S. (1990). "Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods". The Journal of Comparative Neurology. 301 (3): 365–381. doi:10.1002/cne.903010304. PMID 2262596.
- ↑ Altman, J.; Bayer, S. (1990). "Mosaic organization of the hippocampal neuroepithelium and the multiple germinal sources of dentate granule cells". The Journal of Comparative Neurology. 301 (3): 325–342. doi:10.1002/cne.903010302. PMID 2262594.
- ↑ Angevine Jr, J. (1965). "Time of neuron origin in the hippocampal region. An autoradiographic study in the mouse". Experimental neurology. Supplement: Suppl Supp2:1–Supp2. PMID 5838955.
- ↑ Bayer, S. A.; Yackel, J. W.; Puri, P. S. (1982). "Neurons in the rat dentate gyrus granular layer substantially increase during juvenile and adult life". Science. 216 (4548): 890–892. Bibcode:1982Sci...216..890B. doi:10.1126/science.7079742. PMID 7079742.
- ↑ Bayer, S. A. (1982). "Changes in the total number of dentate granule cells in juvenile and adult rats: a correlated volumetric and 3H-thymidine autoradiographic study". Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 46 (3): 315–323. doi:10.1007/bf00238626. PMID 7095040.
- ↑ {Amaral DG., Scharfman HE., Lavenex P. The dentate gyrus: fundamental neuroanatomical organization.Prog Brain Res.2007:163: 3–22.
- ↑ Faiz M, Acarin L, Castellano B, Gonzalez B (2005). "Proliferation dynamics of germinative zone cells in the intact and excitotoxically lesioned postnatal rat brain". BMC Neurosci. 6 (1): 26. doi:10.1186/1471-2202-6-26. PMC 1087489. PMID 15826306.
- 1 2 3 Principles of neural science (5th ed.). Appleton and Lange: McGraw Hill. 2006. ISBN 978-0071390118.
|first1=missing|last1=in Authors list (help) - ↑ Nakashiba, T.; Cushman, J. D.; Pelkey, K. A.; Renaudineau, S.; Buhl, D. L.; McHugh, T. J.; Rodriguez Barrera, V. R.; Chittajallu, R.; Iwamoto, K. S.; McBain, C. J.; Fanselow, M. S.; Tonegawa, S. (2012). "Young Dentate Granule Cells Mediate Pattern Separation, whereas Old Granule Cells Facilitate Pattern Completion". Cell. 149 (1): 188–201. doi:10.1016/j.cell.2012.01.046. PMC 3319279. PMID 22365813.
- ↑ Bliss, Rosalie Marion. "Food and the Aging Mind". First in a Series: Nutrition and Brain Function. http://www.ars.usda.gov/is/ar/archive/aug07/aging0807.htm. (27 February 2010)
- ↑ Malberg JE, Eisch AJ, Nestler EJ, Duman RS (2000). "Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus". J. Neurosci. 20 (24): 9104–9110. PMID 11124987.
- ↑ Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E (1998). "Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress". PNAS. 95 (6): 3168–3171. Bibcode:1998PNAS...95.3168G. doi:10.1073/pnas.95.6.3168. PMC 19713. PMID 9501234.
- ↑ Jacobs B, Praag H, Gage F (2000). "Adult brain neurogenesis and psychiatry: a novel theory of depression". Mol. Psychiatry. 5 (3): 262–9. doi:10.1038/sj.mp.4000712. PMID 10889528.
- ↑ Surget A, Tanti A, Leonardo ED, et al. (December 2011). "Antidepressants recruit new neurons to improve stress response regulation". Molecular Psychiatry. 16 (12): 1177–88. doi:10.1038/mp.2011.48. PMC 3223314. PMID 21537331.
- ↑ Praag, H (1999). "Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus". Nature Neuroscience. 2 (3): 266–270. doi:10.1038/6368. PMID 10195220.
- ↑ Kempermann G, Kuhn HG, Gage FH (1997). "More hippocampal neurons in adult mice living in an enriched environment". Nature. 386 (6624): 493–495. Bibcode:1997Natur.386..493K. doi:10.1038/386493a0. PMID 9087407.
- ↑ Eadie, B.D.; Redilla, VA.; Christie, B.R. (2005). "Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density". The Journal of Comparative Neurology. 486: 39–47. doi:10.1002/cne.20493.
- ↑ http://www.frontiersin.org/Neural_Circuits/researchtopics/Structure_function_and_plastic/737
- ↑ Xavier, GF (2009). "Dentate gyrus and spatial behaviour". Progress in Neuro-Psychopharmacology and Biological Psychiatry. 33 (5): 762–773. doi:10.1016/j.pnpbp.2009.03.036.
- ↑ "Blood Sugar Control Linked to Memory Decline, Study Says". Nytimes.com. 1 January 2009. Retrieved 2011-03-13.
External links
| Wikimedia Commons has media related to Dentate gyrus. |
- Slide at psycheducation.org
- Stained brain slice images which include the "Dentate gyrus" at the BrainMaps project
- "Dentate Gyrus NMDA Receptors Mediate Rapid Pattern Separation in the Hippocampal Network". Science 7 June 2007 doi:10.1126/science.1140263 - The source of déjà vu
- NIF Search - Dentate Gyrus via the Neuroscience Information Framework
- See Altman and Bayer's work on dentate gyrus development and adult neurogenesis